Latest recommendations

| Id | Title▼ | Authors | Abstract | Picture | Thematic fields | Recommender | Reviewers | Submission date | |
|---|---|---|---|---|---|---|---|---|---|
16 Oct 2019

What do ossification sequences tell us about the origin of extant amphibians?Michel Laurin, Océane Lapauze, David Marjanović https://doi.org/10.1101/352609The origins of LissamphibiaRecommended by Robert Asher based on reviews by Jennifer Olori and 2 anonymous reviewersAmong living vertebrates, there is broad consensus that living tetrapods consist of amphibians and amniotes. Crown clade Lissamphibia contains frogs (Anura), salamanders (Urodela) and caecilians (Gymnophiona); Amniota contains Sauropsida (reptiles including birds) and Synapsida (mammals). Within Lissamphibia, most studies place frogs and salamanders in a clade together to the exclusion of caecilians (see Pyron & Wiens 2011). Among fossils, there are a number of amphibian and amphibian-like taxa generally placed in Temnospondyli and Lepospondyli. In contrast to the tree of living tetrapods, affinities of these fossils to some or all of the three extant lissamphibian groups have proven to be much harder to resolve. For example, temnospondyls might be stem tetrapods and lissamphibians a derived group of lepospondyls; alternatively, temnospondyls might be closer to the clade of frogs and salamanders, and lepospondyls to caecilians (compare Laurin et al. 2019: fig. 1d vs. 1f). Here, in order to assess which of these and other mutually exclusive topologies is optimal, Laurin et al. (2019) extract phylogenetic information from developmental sequences, in particular ossification. Several major differences in ossification are known to distinguish vertebrate clades. For example, due to their short intrauterine development and need to climb from the reproductive tract into the pouch, marsupial mammals famously accelerate ossification of their facial skeleton and forelimb; in contrast to placentals, newborn marsupials can climb, smell & suck before they have much in the way of lungs, kidneys, or hindlimbs (Smith 2001). Divergences among living and fossil amphibian groups are likely pre-Triassic (San Mauro 2010; Pyron 2011), much older than a Jurassic split between marsupials and placentals (Tarver et al. 2016), and the quality of the fossil record generally decreases with ever-older divergences. Nonetheless, there are a number of well-preserved examples of "amphibian"-grade tetrapods representing distinct ontogenetic stages (Schoch 2003, 2004; Schoch and Witzmann 2009; Olori 2013; Werneburg 2018; among others), all amenable to analysis of ossification sequences. Putting together a phylogenetic dataset based on ossification sequences is not trivial; sequences are not static features apparent on individual specimens. Rather, one needs multiple specimens representing discrete developmental stages for each taxon to be compared, meaning that sequences are usually available for only a few characters. Laurin et al. (2019) have nonetheless put together the most exhaustive matrix of tetrapod sequences so far, with taxon coverage ranging from 62 genera for appendicular characters to 107 for one of their cranial datasets, each sampling between 4-8 characters (Laurin et al. 2019: table 1). The small number of characters means that simply applying an optimality criterion (such as parsimony) is unlikely to resolve most nodes; treespace is too flat to be able to offer optimal peaks up which a search algorithm might climb. However, Laurin et al. (2019) were able to test each of the main competing hypotheses, defined a priori as a branching topology, given their ossification sequence dataset and a likelihood optimality criterion. Their most consistent result comes from their cranial ossification sequences and supports their "LH", or lepospondyl hypothesis (Laurin et al. 2019: fig. 1d). That is, relative to extinct, "amphibian"-grade taxa, Lissamphibia is monophyletic and nested within lepospondyls. Compared to mammals and birds (including dinosaurs), crown amphibian branches of the Tree of Life are exceptionally old. Each lissamphibian clade likely had diverged during Permian times (Marjanovic & Laurin 2008) and the crown group itself may even date to the Carboniferous (Pyron 2011). In contrast to mammoths and moas, no ancient DNA or collagen sequences are going to be available from >300 million-year-old fossils like the lepospondyl *Hyloplesion* (Olori 2013), although recently published methods for incorporating genomic signal from extant taxa (Beck & Baillie 2018; Asher et al. 2019) into studies of fossils could also be applied to these ancient divergences among amphibian-grade tetrapods. Ossification sequences represent another important, additional source of data with which to test the conclusion of Laurin et al. (2019) that monophyletic Lissamphibians shared a common ancestor with lepospondyls, among other hypotheses. **References** Asher, R. J., Smith, M. R., Rankin, A., & Emry, R. J. (2019). Congruence, fossils and the evolutionary tree of rodents and lagomorphs. Royal Society Open Science, 6(7), 190387. doi: [ 10.1098/rsos.190387 ](https://dx.doi.org/ 10.1098/rsos.190387 ) Beck, R. M. D., & Baillie, C. (2018). Improvements in the fossil record may largely resolve current conflicts between morphological and molecular estimates of mammal phylogeny. Proceedings of the Royal Society B: Biological Sciences, 285(1893), 20181632. doi: [ 10.1098/rspb.2018.1632](https://dx.doi.org/ 10.1098/rspb.2018.1632) Laurin, M., Lapauze, O., & Marjanović, D. (2019). What do ossification sequences tell us about the origin of extant amphibians? BioRxiv, 352609, ver. 4 peer-reviewed by PCI Paleo. doi: [ 10.1101/352609](https://dx.doi.org/ 10.1101/352609) Marjanović, D., & Laurin, M. (2008). Assessing confidence intervals for stratigraphic ranges of higher taxa: the case of Lissamphibia. Acta Palaeontologica Polonica, 53(3), 413–432. doi: [ 10.4202/app.2008.0305](https://dx.doi.org/ 10.4202/app.2008.0305) Olori, J. C. (2013). Ontogenetic sequence reconstruction and sequence polymorphism in extinct taxa: an example using early tetrapods (Tetrapoda: Lepospondyli). Paleobiology, 39(3), 400–428. doi: [ 10.1666/12031](https://dx.doi.org/ 10.1666/12031) Pyron, R. A. (2011). Divergence time estimation using fossils as terminal taxa and the origins of Lissamphibia. Systematic Biology, 60(4), 466–481. doi: [ 10.1093/sysbio/syr047](https://dx.doi.org/ 10.1093/sysbio/syr047) Pyron, R. A., & Wiens, J. J. (2011). A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Molecular Phylogenetics and Evolution, 61(2), 543–583. doi: [ 10.1016/j.ympev.2011.06.012](https://dx.doi.org/ 10.1016/j.ympev.2011.06.012) San Mauro, D. (2010). A multilocus timescale for the origin of extant amphibians. Molecular Phylogenetics and Evolution, 56(2), 554–561. doi: [ 10.1016/j.ympev.2010.04.019](https://dx.doi.org/ 10.1016/j.ympev.2010.04.019) Schoch, R. R. (2003). Early larval ontogeny of the Permo-Carboniferous temnospondyl *Sclerocephalus*. Palaeontology, 46(5), 1055–1072. doi: [ 10.1111/1475-4983.00333](https://dx.doi.org/ 10.1111/1475-4983.00333) Schoch, R. R. (2004). Skeleton formation in the Branchiosauridae: a case study in comparing ontogenetic trajectories. Journal of Vertebrate Paleontology, 24(2), 309–319. doi: [ 10.1671/1950](https://dx.doi.org/ 10.1671/1950) Schoch, R. R., & Witzmann, F. (2009). Osteology and relationships of the temnospondyl genus *Sclerocephalus*. Zoological Journal of the Linnean Society, 157(1), 135–168. doi: [ 10.1111/j.1096-3642.2009.00535.x](https://dx.doi.org/ 10.1111/j.1096-3642.2009.00535.x) Smith, K. K. (2001). Heterochrony revisited: the evolution of developmental sequences. Biological Journal of the Linnean Society, 73(2), 169–186. doi: [ 10.1111/j.1095-8312.2001.tb01355.x](https://dx.doi.org/ 10.1111/j.1095-8312.2001.tb01355.x) Tarver, J. E., dos Reis, M., Mirarab, S., Moran, R. J., Parker, S., O’Reilly, J. E., & Pisani, D. (2016). The interrelationships of placental mammals and the limits of phylogenetic inference. Genome Biology and Evolution, 8(2), 330–344. doi: [ 10.1093/gbe/evv261](https://dx.doi.org/ 10.1093/gbe/evv261) Werneburg, R. (2018). Earliest “nursery ground” of temnospondyl amphibians in the Permian. Semana, 32, 3–42. | What do ossification sequences tell us about the origin of extant amphibians? | Michel Laurin, Océane Lapauze, David Marjanović | <p>The origin of extant amphibians has been studied using several sources of data and methods, including phylogenetic analyses of morphological data, molecular dating, stratigraphic data, and integration of ossification sequence data, but a consen... |  | Evo-Devo, Phylogenetics, Systematics, Vertebrate paleontology | Robert Asher | 2018-06-22 08:21:31 | View | |
23 Apr 2021

The record of Deinotheriidae from the Miocene of the Swiss Jura Mountains (Jura Canton, Switzerland)Fanny Gagliardi, Olivier Maridet, Damien Becker https://doi.org/10.1101/2020.08.10.244061The fossil record of deinotheres in the Jura Mountains and the specific diversity of European deinotheriidsRecommended by Lionel Hautier based on reviews by Martin Pickford and 1 anonymous reviewerProboscideans belong to the Afrotheria, a superorder of mammals with an African origin, which was recently recognized based on molecular data (see review in Asher et al., 2009). The fossil record of Proboscidea is well documented and shows that an important part of their evolutionary history took place in Africa, with their representatives inhabiting the continent for at least 60 million years (Gheerbrant, 2009). However, proboscideans also proved to be great travellers, and a flourishing diversity of proboscidean forms colonized most of the continents of the planet, including Europe, from where they have since completely disappeared. Nowadays, Loxodonta africana, L. cyclotis, and Elephas maximus are flagship species of the African and Asian faunas, but they only represent a minor part of the modern mammalian diversity. In contrast, their ancient relatives seemed to be relatively abundant in past ecosystems (Sanders et al., 2010), which raised a number of interesting, but challenging, questions relative to the structure and evolution of ancient megaherbivore communities (Calandra et al., 2008). Among proboscideans, deinotheres represent a special case. Their morphology clearly departs from that of other groups, notably in displaying distinctive downward curving lower tusks. Compared to their successful sister group the elephantiforms (i.e., all elephant-like proboscideans closely related to modern elephants; sensu Tassy, 1994), deinotheriids are often regarded as the poor sibling of the Proboscidea for showing a relatively low specific diversity and displaying a reduced morphological variability. In fact, many grey areas still exist regarding the evolution of this unique family. In their article, Gagliardi et al. (2021) revised the material of deinotheres recovered in the Miocene sands of the Swiss Jura Mountains. They described for the first time the material attributed to Prodeinotherium bavaricum and Deinotherium giganteum from the Delémont valley, and reported the presence of a third species, Deinotherium levius, from the locality of Charmoille in Ajoie. Based on comparisons made on specimens recovered from middle to the late Miocene localities, the authors discussed the potential link between the mode and tempo of deinothere dispersions and the evolution environmental and climatic conditions in Western and Eastern Europe during the Miocene. They also considered the evolution of ecological specializations in the group, especially with regard to size increase. Gagliardi et al. (2021) proposed to follow the two genera/five species concept (i.e., P. cuvieri, P. bavaricum, D. levius, D. giganteum, and D. proavum), which implies the co-existence of several deinothere species in Europe. The latter hypothesis contrasts with the recognition of a single African Deinotherium species (i.e., D. bozasi) in deposits dated from the late Miocene to the early Pleistocene (Sanders et al., 2010). Such a co-existence of European species was and still is debated; it was here questioned by both reviewers. However, as acknowledged by the authors, only an extensive revision of the material of all recognized species, in Europe and worldwide, will enable to shed more light on the deinothere morphological variability and specific diversity. There is no doubt that such a revision would have a profound impact on our view of the evolution of this enigmatic group.
References Asher, R. J., Bennett, N., & Lehmann, T. (2009). The new framework for understanding placental mammal evolution. BioEssays, 31(8), 853–864. doi: 10.1002/bies.200900053 Calandra, I., Göhlich, U. B., & Merceron, G. (2008). How could sympatric megaherbivores coexist? Example of niche partitioning within a proboscidean community from the Miocene of Europe. Naturwissenschaften, 95(9), 831–838. doi: 10.1007/s00114-008-0391-y Gagliardi, F., Maridet, O., & Becker, D. (2021). The record of Deinotheriidae from the Miocene of the Swiss Jura Mountains (Jura Canton, Switzerland). BioRxiv, 244061, ver. 4 peer-reviewed by PCI Paleo. doi: 10.1101/2020.08.10.244061 Gheerbrant, E. (2009). Paleocene emergence of elephant relatives and the rapid radiation of African ungulates. Proceedings of the National Academy of Sciences, 106(26), 10717–10721. doi: 10.1073/pnas.0900251106 Sanders, W. J., Gheerbrant, E., Harris, J. M., Saegusa, H., & Delmer, C. (2010). Proboscidea. In L. Werdelin & W. J. Sanders (Eds.), Cenozoic Mammals of Africa (pp. 161–251). Berkeley: University of California Press. doi: 10.1525/california/9780520257214.003.0015 Tassy, P. (1994). Origin and differentiation of the Elephantiformes (Mammalia, Proboscidea). Verhandlungen Naturwissenschaftlichen Vereins in Hamburg, 34, 73–94. | The record of Deinotheriidae from the Miocene of the Swiss Jura Mountains (Jura Canton, Switzerland) | Fanny Gagliardi, Olivier Maridet, Damien Becker | <p>The Miocene sands of the Swiss Jura Mountains, long exploited in quarries for the construction industry, have yielded abundant fossil remains of large mammals. Among Deinotheriidae (Proboscidea), two species, Prodeinotherium bavaricum and Deino... |  | Fossil record, Paleobiogeography, Taxonomy, Vertebrate paleontology | Lionel Hautier | 2020-08-11 10:17:38 | View | |
19 Aug 2021

Through a glass darkly, but with more understanding of arthropod originRecommended by Tae-Yoon Park based on reviews by Gerhard Scholtz and Jean VannierArthropods constitute 85% of all described animal species on Earth (Brusca et al., 2016), being the most successful animal phylum at present. This phylum did not even have a shabby beginning. The fossil record shows that they were dominating the sea even in the early Cambrian (Caron and Jackson, 2008; Zhao et al., 2014; Fu et al., 2019). This planet is and has been indeed dominated by arthropods. Within the context of the Big Bang of animal evolution known as the Cambrian Explosion, delving into the origin of arthropods has been one of the all-time fascinating research themes in paleontology, and discussions on the ‘origin’ and ‘early evolution’ of arthropods from the paleontological perspective have not been infrequent (e.g., Budd and Telford, 2009; Edgecombe and Legg, 2014; Daley et al., 2018; Edgecombe, 2020). In this context, Aria (2021) provides an interesting integration of his view on arthropod origin and early evolution. Based on well-preserved Burgess Shale materials, together with other impressive researches mainly with Jean-Bernard Caron (e.g., Aria and Caron, 2015; Caron and Aria, 2017), Cédric Aria made his name known with papers searching for the stem-groups of the two major euarthropod lineages, the Mandibulata and the Chelicerata (Aria and Caron, 2017, 2019), for which (and for his subsequent researches) assembling a large dataset for arthropod phylogeny was inevitable. Subsequently, there have been several major discoveries which could improve our understanding on early arthropod evolution (e.g., Lev and Chipman, 2020; Liu et al., 2020; Zeng et al., 2020). For Cédric Aria, therefore, this timely presentation of his own perspective on the origin of early evolution of arthropods may have been preordain. This review stands out because it discusses almost all aspects of arthropod origin and early evolution that can be possibly covered by paleontology (many, if not all, of which are still controversial). Some of his views may be considered a brave attempt. For instance, based on the widespread occurrences of suspension feeders, Aria (2021) proposes the early Cambrian “planktonic revolution,” which has been associated rather with the Great Ordovician Biodiversification Event (Servais et al., 2016). Given the presence of lophotrochozoans and echinoderms in which the presence of planktonic larvae was likely to have been one of the most inclusive features, the “early Cambrian planktonic revolution” might be plausible, but I wonder if including arthropods into the “earlier revolution” can be readily acceptable. Arthropods arose from direct-developing ancestors, and crustaceans (the main group with planktonic larvae) are pretty much derived in the arthropod phylogenetic tree. Trilobites, inarguably the best-studied Cambrian arthropods, for example, began with direct-developing benthic protaspides in the early Cambrian and Miaolingian. The first hint of planktonic protaspides appeared in the Furongian, and it was not until the Tremadocian when such indirect developing protaspides began to be widespread (Park and Kihm, 2015), which complies well with the onset of the ‘Ordovician Planktonic Revolution’ in the Furongian, as suggested by Servais et al. (2016). But in general, this review presents well-organized views worth hearing, and since many of the points are subject to debate, this review could be a friendly manual for those who have similar views, while it could form a fresh ground to attack for those who have disparate views. One of the endless debates in arthropod research comes from the arthropod head problem (Budd, 2002), which centers on the homology of frontal-most appendages in radiodonts, megacheirans, chelicerates, and mandibulates, as well as on the hypostome-labrum complex. Based on recent interesting discoveries of early arthropods from the Chengjiang biota (Aria, 2020; Zeng et al., 2020), Aria (2021) pertinently coined a term ‘cheirae’ for the frontalmost prehensile appendages of radiodonts and megacheirans, implying homology of them. I assume that not all researchers would agree with this though, as well as with his model of hypostome/labrum complex evolution. Notorious disaccords have also occurred around the phylogenetic positions of early arthropods, such as isoxyids, megacheirans, fuxianhuiids, and artiopodans; different research groups have invariably come up with different topologies. Cédric Aria has presented his own topologies (Aria, 2019, 2020), and figure 2 of Aria (2021) summarizes his perspective very well in combination with major character evolutions. What makes this review especially interesting is a courageous discussion about the origin of biramous appendage, a subject that has been only briefly discussed or overlooked in recent literature. In the current mainstream of this debate lies the concept of ‘gilled lobopodians’ (Budd, 1998), which was complicated by the weird two separate rows of lateral flaps of Aegirocassis (Van Roy et al., 2015). Aria (2021) adds an interesting alternative scenario to this: biramicity originated from the split of main limb axis, as seen in the isoxyid Surusicaris (Aria and Caron, 2015). The figure 3d of his review, therefore, is worth giving thoughts for any arthropodologists who are interested in the origin of arthropod legs. It is true that our understanding of the origin and early evolution of arthropods is still in a mist. Nevertheless, we have recently seen advancements, such as those aided by new types of analysis (Liu et al., 2020), the discoveries of new early arthropods with unexpected morphologies (Aria et al., 2020; Zeng et al., 2020), and the groundbreaking Evo-Devo research (Lev and Chipman, 2020). We will keep jousting on various aspects of the origin and early evolution of arthropods, but for some aspects we are seemingly heading for the final, as implicitly alluded in Aria (2021).
References Aria C (2019). Reviewing the bases for a nomenclatural uniformization of the highest taxonomic levels in arthropods. Geological Magazine 156, 1463–1468. Aria C (2020). Macroevolutionary patterns of body plan canalization in euarthropods. Paleobiology 46, 569–593. doi: 10.1017/pab.2020.36 Aria C (2021). The origin and early evolution of arthropods. PaleorXiv, 4zmey, ver. 4, peer-reviewed by PCI Paleo. doi: 10.31233/osf.io/4zmey Aria C and Caron JB (2015). Cephalic and limb anatomy of a new isoxyid from the Burgess Shale and the role of "stem bivalved arthropods" in the disparity of the frontalmost appendage. PLOS ONE 10, e0124979. doi: 10.1371/journal.pone.0124979 Aria C and Caron JB (2017). Burgess Shale fossils illustrate the origin of the mandibulate body plan. Nature 545, 89–92. Aria C and Caron JB (2019). A middle Cambrian arthropod with chelicerae and proto-book gills. Nature 573, 586–589. doi: 10.1038/s41586-019-1525-4 Aria C, Zhao F, Zeng H, Guo J, and Zhu M (2020). Fossils from South China redefine the ancestral euarthropod body plan. BMC Evolutionary Biology 20, 4. Brusca RC, Moore W, and Shuster SM (2016). Invertebrates. Third edition. Sunderland, Massachusetts U.S.A: Sinauer Associates. isbn: 978-1-60535-375-3 Budd GE (1998). Stem-group arthropods from the Lower Cambrian Sirius Passet fauna of North Greenland. In: Arthropod Relationships. Ed. by Fortey RA and Thomas RH. London, UK: Chapman & Hall, pp. 125–138. Budd GE (2002). A palaeontological solution to the arthropod head problem. Nature 417, 271–275. doi: 10.1038/417271a Budd GE and Telford MJ (2009). The origin and evolution of arthropods. Nature 457, 812–817. doi: 10.1038/Nature07890 Caron JB and Aria C (2017). Cambrian suspension-feeding lobopodians and the early radiation of panarthropods. BMC Evolutionary Biology 17, 29. doi: 10.1186/s12862-016-0858-y Caron JB and Jackson DA (2008). Paleoecology of the Greater Phyllopod Bed community, Burgess Shale. Palaeogeography, Palaeoclimatology, Palaeoecology 258, 222–256. doi: 10.1016/j.palaeo.2007.05.023 Daley AC, Antcliffe JB, Drage HB, and Pates S (2018). Early fossil record of Euarthropoda and the Cambrian Explosion. Proceedings of the National Academy of Sciences of the United States of America 115, 5323–5331. doi: 10.1073/pnas.1719962115 Edgecombe GD (2020). Arthropod origins: Integrating paleontological and molecular evidence. Annual Review of Ecology, Evolution, and Systematics 51, 1–25. doi: 10.1146/annurev-ecolsys-011720-124437 Edgecombe GD and Legg DA (2014). Origins and early evolution of arthropods. Palaeontology 57, 457–468. Fu D, Tong G, Dai T, Liu W, Yang Y, Zhang Y, Cui L, Li L, Yun H, Wu Y, Sun A, Liu C, Pei W, Gaines RR, and Zhang X (2019). The Qingjiang biota—A Burgess Shale–type fossil Lagerstätte from the early Cambrian of South China. Science 363, 1338–1342. doi: 10.1126/science.aau8800 Lev O and Chipman AD (2020). Development of the pre-gnathal segments of the insect head indicates they are not serial homologues of trunk segments. bioRxiv, 2020.09.16.299289. doi: 10.1101/2020.09.16.299289 Liu Y, Ortega-Hernández J, Zhai D, and Hou X (2020). A reduced labrum in a Cambrian great-appendage euarthropod. Current Biology 30, 3057–3061.e2. doi: 10.1016/j.cub.2020.05.085 Park TYS and Kihm JH (2015). Post-embryonic development of the Early Ordovician (ca. 480 Ma) trilobite Apatokephalus latilimbatus Peng, 1990 and the evolution of metamorphosis. Evolution & Development 17, 289–301. doi: 10.1111/ede.12138 Servais T, Perrier V, Danelian T, Klug C, Martin R, Munnecke A, Nowak H, Nützel A, Vandenbroucke TR, Williams M, and Rasmussen CM (2016). The onset of the ‘Ordovician Plankton Revolution’ in the late Cambrian. Palaeogeography, Palaeoclimatology, Palaeoecology 458, 12–28. doi: 10.1016/j.palaeo.2015.11.003 Van Roy P, Daley AC, and Briggs DEG (2015). Anomalocaridid trunk limb homology revealed by a giant filter-feeder with paired flaps. Nature 522, 77–80. doi: 10.1038/nature14256 Zeng H, Zhao F, Niu K, Zhu M, and Huang D (2020). An early Cambrian euarthropod with radiodont-like raptorial appendages. Nature 588, 101–105. doi: 10.1038/s41586-020-2883-7 Zhao F, Caron JB, Bottjer DJ, Hu S, Yin Z, and Zhu M (2014). Diversity and species abundance patterns of the Early Cambrian (Series 2, Stage 3) Chengjiang Biota from China. Paleobiology 40, 50–69. doi: 10.1666/12056 | The origin and early evolution of arthropods | Cédric Aria | <p style="text-align: justify;">The rise of arthropods is a decisive event in the history of life. Likely the first animals to have established themselves on land and in the air, arthropods have pervaded nearly all ecosystems and have become pilla... |  | Comparative anatomy, Evo-Devo, Evolutionary biology, Fossil record, Invertebrate paleontology, Macroevolution, Paleobiodiversity, Paleobiology, Paleoecology, Phylogenetics, Systematics, Taphonomy, Taxonomy | Tae-Yoon Park | 2021-04-26 13:51:21 | View | |
30 Oct 2019

The Morrison Formation Sauropod Consensus: A freely accessible online spreadsheet of collected sauropod specimens, their housing institutions, contents, references, localities, and other potentially useful informationEmanuel Tschopp, John A. Whitlock, D. Cary Woodruff, John R. Foster, Roberto Lei, Simone Giovanardi https://doi.org/10.31233/osf.io/ydvraSauropods under one (very high) roofRecommended by Jordan Mallon based on reviews by Kenneth Carpenter and Femke HolwerdaFossils get around. Any one fossil locality might be sampled by several collectors from as many institutions around the world. Alternatively, a single collector might heavily sample a site, and sell or trade parts of their collection to other institutions, scattering the fossils far and wide. These practices have the advantage of making fossils from any one locality available to researchers across the globe. However, they also have the disadvantage that, in order to systematically survey any one species, a researcher must follow innumerable trails of breadcrumb to get to where the relevant materials are held. This is true of many famous fossil localities, such as the Eocene Green River Formation in the USA, the Cretaceous Kem Kem beds of Morocco, or the Devonian Miguasha cliffs of Canada. It is especially true of the Upper Jurassic deposits of the Morrison Formation in the western USA, which have yielded an impressive assemblage of megaherbivorous sauropod dinosaurs over the last 150 years. Today, these bones are to be found in museums not just in the USA, but also in Canada, Argentina, Japan, Australia, Malaysia, South Africa, and throughout Europe. Trawling museum databases in search of sauropod material from the Morrison Formation can therefore be a daunting task, never mind traveling the globe to actually study them. A new paper by Tschopp et al. (2019) seeks to ease the burden on sauropod researchers by introducing a database of Morrison Formation sauropods, consisting of over 3000 specimens housed in nearly 40 institutions around the world. The authors are themselves sauropod workers and, having suffered first-hand the plight of studying material from the Morrison Formation, came up with a solution to the problem of keeping track of it all. The database is founded largely on material personally seen by the authors, supplemented by information from the literature and museum catalogs. The database further provides information on bone representation, ontogeny, locality details, and fine-scale stratigraphy, among other fields. Like any database, it is a living document that will continue to grow as new finds are made. Tschopp et al. (2019) have wisely chosen to allow others to contribute to the listing, but changes must first be vetted for accuracy. This product represents 10 years of work, and I have little doubt that it will be well-received by those of us who work on dinosaurs. Speaking personally, my PhD research on megaherbivorous dinosaurs from the Dinosaur Park Formation of Canada led me to institutions in Canada, the USA, and the UK, and further stops to Spain and Argentina would have been beneficial, if affordable. Planning for this work would have been greatly assisted by a database like the one provided us by Tschopp et al. (2019). Many a future graduate student will undoubtedly owe them a debt of gratitude. References Tschopp, E., Whitlock, J. A., Woodruff, D. C., Foster, J. R., Lei, R., & Giovanardi, S. (2019). The Morrison Formation Sauropod Consensus: A freely accessible online spreadsheet of collected sauropod specimens, their housing institutions, contents, references, localities, and other potentially useful information. PaleorXiv, version 3, peer-reviewed by PCI Paleo. doi: 10.31233/osf.io/ydvra | The Morrison Formation Sauropod Consensus: A freely accessible online spreadsheet of collected sauropod specimens, their housing institutions, contents, references, localities, and other potentially useful information | Emanuel Tschopp, John A. Whitlock, D. Cary Woodruff, John R. Foster, Roberto Lei, Simone Giovanardi | <p>The Morrison Formation has been explored for dinosaurs for more than 150 years, in particular for large sauropod skeletons to be mounted in museum exhibits around the world. Several long-term campaigns to the Jurassic West of the United States ... |  | Fossil record, Methods, Paleobiodiversity, Taxonomy, Vertebrate paleontology | Jordan Mallon | 2019-07-19 16:13:45 | View | |
27 May 2020

The last surviving Thalassochelydia—A new turtle cranium from the Early Cretaceous of the Purbeck Group (Dorset, UK)Jérémy Anquetin, Charlotte André https://doi.org/10.31233/osf.io/7pa5cA recommendation of: The last surviving Thalassochelydia—A new turtle cranium from the Early Cretaceous of the Purbeck Group (Dorset, UK)Recommended by Hans-Dieter Sues based on reviews by Igor Danilov and Serjoscha EversStem- and crown-group turtles have a rich and varied fossil record dating back to the Triassic Period. By far the most common remains of these peculiar reptiles are their bony shells and fragments of shells. Furthermore, if historical specimens preserved skulls the preparation techniques at that time were inadequate for elucidating details of the cranial structure. Thus, it comes as no surprise that most of the early research on turtles focused on the structure of the shell with little attention paid to other parts of the skeleton. Starting in the 1960s, this changed as researchers realized that there is considerable variation in the structure of turtle shells even within species and that new methods of fossil preparation, especially chemical methods, could reveal a wealth of phylogenetically important features in the structure of the skulls of turtles. The principal worker was Eugene S. Gaffney of the American Museum of Natural History (New York) who in a series of exquisitely illustrated monographs revolutionized our understanding of turtle osteology and phylogeny. Over the last decade or so, a new generation of researchers has further refined the phylogenetic framework for turtles and continued the work by Gaffney. One of the specialists from this new generation is Jérémy Anquetin who, with a number of colleagues, has revised many of the Jurassic-age stem-turtles that existed in coastal marine settings in what is now Europe. Collections in France, Germany, Switzerland, and the UK house numerous specimens of these forms, which attracted the interest of researchers as early as the first decades of the nineteenth century. Despite this long history, however, the diversity and interrelationships of these marine taxa remained poorly understood. In the present study, Anquetin and his colleague Charlotte André extend the fossil record of these stem-turtles, recently hypothesized as a clade Thalassochelydia, into the Early Cretaceous (Anquetin & André 2020). They present an excellent anatomical account on a well-preserved cranium from the Purbeck Formation of Dorset (England) that can be referred to Thalassochelydia and augments our knowledge of the cranial morphology of this clade. Anquetin & André (2020) make a good case that this specimen belongs to the same taxon as shell material long ago described as Hylaeochelys belli. References Anquetin, J., & André, C. (2020). The last surviving Thalassochelydia—A new turtle cranium from the Early Cretaceous of the Purbeck Group (Dorset, UK). PaleorXiv, 7pa5c, version 3, peer-reviewed by PCI Paleo. doi: 10.31233/osf.io/7pa5c | The last surviving Thalassochelydia—A new turtle cranium from the Early Cretaceous of the Purbeck Group (Dorset, UK) | Jérémy Anquetin, Charlotte André | <p>**Background.** The mostly Berriasian (Early Cretaceous) Purbeck Group of southern England has produced a rich turtle fauna dominated by the freshwater paracryptodires *Pleurosternon bullockii* and *Dorsetochelys typocardium*. Each of these spe... |  | Comparative anatomy, Paleoecology, Phylogenetics, Systematics, Vertebrate paleontology | Hans-Dieter Sues | 2020-01-30 10:37:07 | View | |
14 Apr 2021
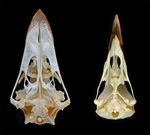
The impact of allometry on vomer shape and its implications for the taxonomy and cranial kinesis of crown-group birdsOlivia Plateau, Christian Foth https://doi.org/10.1101/2020.07.02.184101Vomers aren't so different in crown group birds when considering allometric effectsRecommended by Andrew Farke based on reviews by Sergio Martínez Nebreda and Roland SookiasToday’s birds are divided into two deeply divergent and historically well-documented groups: Palaeognathae and Neognathae. Palaeognaths include both the flight-capable tinamous as well as the flightless ratites (ostriches, rheas, kiwis, cassowaries, and kin). Neognaths include all other modern birds, ranging from sparrows to penguins to hummingbirds. The clade names refer to the anatomy of the palate, with the “old jaws” (palaeognaths) originally thought to more closely resemble an ancestral reptilian condition and the “new jaws” (neognaths) showing a uniquely modified bony configuration. This particularly manifests in the pterygoid-palatine complex (PPC) in the palate, formed from pairs of pterygoids and palatines alongside a single midline vomer. In palaeognaths, the vomer is comparatively large and the pterygoid and palatine are relatively tightly connected. The PPC is more mobile in neognaths, with a variably shaped vomer, which is sometimes even absent. Although both groups of birds show cranial kinesis, neognaths exhibit a much more pronounced degree of kinesis versus palaeognaths, due in part to the tighter nature of the palaeognath pterygoid/palatine interfaces. A previous paper (Hu et al. 2019) used 3D geometric morphometrics to compare the shape of the vomer across neognaths and palaeognaths. Among other findings, this work suggested that each clade had a distinct vomer morphology, with palaeognaths more similar to the ancestral condition (i.e., that of non-avian dinosaurs). This observation was extended to support inferences of limited vs. less limited cranial kinesis in various extinct species, based in part on observations of vomer shape. A new preprint by Plateau and Foth (2021) presents a reanalysis of Hu et al.’s data, specifically focusing on allometric effects. In short, the new analysis looks at how size correlates (or doesn't correlate) with vomer shape. Plateau and Foth (2021) found that when size effects are included, differences between palaeognaths and neognaths are less than the “raw” (uncorrected) shape data suggest. It is much harder to tell bird groups apart! Certainly, there are still some general differences, but some separations in morphospace close up when allometry—the interrelationship between shape and size—is considered. Plateau and Foth (2021) use this finding to suggest that 1) vomer shape alone is not a completely reliable proxy for inferring the phylogenetic affinities of a particular bird; and 2) the vomer is only one small component of the cranial kinetic system, and thus its shape is of limited utility for inferring cranial kinesis capabilities when considered independently from the rest of the relevant skull bones.
References Hu, H., Sansalone, G., Wroe, S., McDonald, P. G., O’Connor, J. K., Li, Z., Xu, X., & Zhou, Z. (2019). Evolution of the vomer and its implications for cranial kinesis in Paraves. Proceedings of the National Academy of Sciences, 116(39), 19571–19578. doi: 10.1073/pnas.1907754116 | The impact of allometry on vomer shape and its implications for the taxonomy and cranial kinesis of crown-group birds | Olivia Plateau, Christian Foth | <p>Crown birds are subdivided into two main groups, Palaeognathae and Neognathae, that can be distinguished, among others, by the organization of the bones in their pterygoid-palatine complex (PPC). Shape variation to the vomer, which is the most ... |  | Comparative anatomy, Evolutionary biology, Macroevolution, Morphological evolution, Morphometrics, Taxonomy | Andrew Farke | 2020-07-03 14:16:48 | View | |
15 Dec 2022
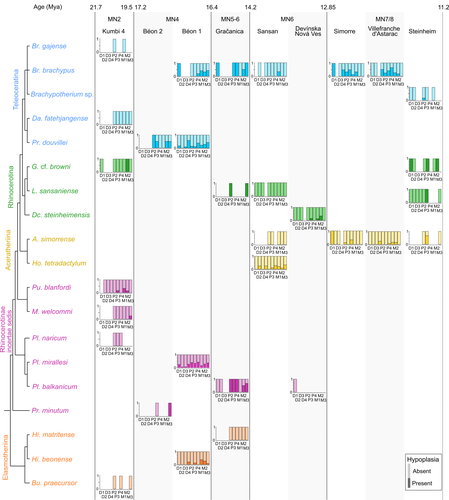
Spatio-temporal diversity of dietary preferences and stress sensibilities of early and middle Miocene Rhinocerotidae from Eurasia: impact of climate changesManon Hullot, Gildas Merceron, Pierre-Olivier Antoine https://doi.org/10.1101/2022.05.06.490903New insights into the palaeoecology of Miocene Eurasian rhinocerotids based on tooth analysisRecommended by Alexandra Houssaye based on reviews by Antigone Uzunidis, Christophe Mallet and Matthew MihlbachlerRhinocerotoidea originated in the Lower Eocene and diversified well during the Cenozoic in Eurasia, North America and Africa. This taxon encompasses a great diversity of ecologies and body proportions and masses. Within this group, the family Rhinocerotidae, which is the only one with extant representatives, appeared in the Late Eocene (Prothero & Schoch, 1989). They were well diversified during the Early and Middle Miocene, whereas they began to decline in both diversity and geographical range after the Miocene, throughout the Pliocene and Pleistocene, in conjunction with the marked climatic changes (Cerdeño, 1998). In Eurasian Early and Middle Miocene fossil localities, a variety of species are often associated. Therefore, it may be quite difficult to estimate how these large herbivores cohabited and whether competition for food resources is reflected in a diversity of ecological niches. The ecologies of these large mammals are rather poorly known and the detailed study of their teeth could bring new elements of answer. Indeed, if teeth carry a strong phylogenetic signal in mammals, they are also of great interest for ecological studies, and they have the additional advantage of being often numerous in the fossil record. Hullot et al. (2022) analysed both dental microwear texture, as an indicator of dietary preferences, and enamel hypoplasia, to identify stress sensitivity, in a large number of rhinocerotid fossil teeth from nine Neogene (Early to Middle Miocene) localities in Europe and Pakistan. Their aim was to analyse whether fossil species diversity is associated with a diversity of ecologies, and to investigate possible ecological differences between regions and time periods in relation to climate change. Their results show clear differences in time and space between and within species, and suggest that more flexible species are less vulnerable to environmental stressors. Very few studies focus on the palaeocology of Miocene rhinos. This study is therefore a great contribution to the understanding of the evolution of this group.
References Cerdeño, E. (1998). Diversity and evolutionary trends of the Family Rhinocerotidae (Perissodactyla). Palaeogeography, Palaeoclimatology, Palaeoecology, 141, 13–34. https://doi.org/10.1016/S0031-0182(98)00003-0 Hullot, M., Merceron, G., and Antoine, P.-O. (2022). Spatio-temporal diversity of dietary preferences and stress sensibilities of early and middle Miocene Rhinocerotidae from Eurasia: Impact of climate changes. BioRxiv, 490903, ver. 4 peer-reviewed by PCI Paleo. https://doi.org/10.1101/2022.05.06.490903 Prothero, D. R., and Schoch, R. M. (1989). The evolution of perissodactyls. New York: Oxford University Press. | Spatio-temporal diversity of dietary preferences and stress sensibilities of early and middle Miocene Rhinocerotidae from Eurasia: impact of climate changes | Manon Hullot, Gildas Merceron, Pierre-Olivier Antoine | <p>Major climatic and ecological changes are documented in terrestrial ecosystems during the Miocene epoch. The Rhinocerotidae are a very interesting clade to investigate the impact of these changes on ecology, as they are abundant and diverse in ... |  | Paleobiodiversity, Paleobiology, Paleoecology, Paleopathology, Vertebrate paleontology | Alexandra Houssaye | 2022-05-09 09:33:30 | View | |
19 Sep 2023
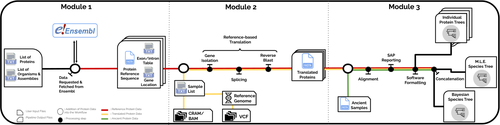
PaleoProPhyler: a reproducible pipeline for phylogenetic inference using ancient proteinsIoannis Patramanis, Jazmín Ramos-Madrigal, Enrico Cappellini, Fernando Racimo https://doi.org/10.1101/2022.12.12.519721An open-source pipeline to reconstruct phylogenies with paleoproteomic dataRecommended by Leslea Hlusko based on reviews by Katerina Douka and 2 anonymous reviewersOne of the most recent technological advances in paleontology enables the characterization of ancient proteins, a new discipline known as palaeoproteomics (Ostrom et al., 2000; Warinner et al., 2022). Palaeoproteomics has superficial similarities with ancient DNA, as both work with ancient molecules, however the former focuses on peptides and the latter on nucleotides. While the study of ancient DNA is more established (e.g., Shapiro et al., 2019), palaeoproteomics is experiencing a rapid diversification of application, from deep time paleontology (e.g., Schroeter et al., 2022) to taxonomic identification of bone fragments (e.g., Douka et al., 2019), and determining genetic sex of ancient individuals (e.g., Lugli et al., 2022). However, as Patramanis et al. (2023) note in this manuscript, tools for analyzing protein sequence data are still in the informal stage, making the application of this methodology a challenge for many new-comers to the discipline, especially those with little bioinformatics expertise. In the spirit of democratizing the field of palaeoproteomics, Patramanis et al. (2023) developed an open-source pipeline, PaleoProPhyler released under a CC-BY license (https://github.com/johnpatramanis/Proteomic_Pipeline). Here, Patramanis et al. (2023) introduce their workflow designed to facilitate the phylogenetic analysis of ancient proteins. This pipeline is built on the methods from earlier studies probing the phylogenetic relationships of an extinct genus of rhinoceros Stephanorhinus (Cappellini et al., 2019), the large extinct ape Gigantopithecus (Welker et al., 2019), and Homo antecessor (Welker et al., 2020). PaleoProPhyler has three interacting modules that initialize, construct, and analyze an input dataset. The authors provide a demonstration of application, presenting a molecular hominid phyloproteomic tree. In order to run some of the analyses within the pipeline, the authors also generated the Hominid Palaeoproteomic Reference Dataset which includes 10,058 protein sequences per individual translated from publicly available whole genomes of extant hominids (orangutans, gorillas, chimpanzees, and humans) as well as some ancient genomes of Neanderthals and Denisovans. This valuable research resource is also publicly available, on Zenodo (Patramanis et al., 2022). Three reviewers reported positively about the development of this program, noting its importance in advancing the application of palaeoproteomics more broadly in paleontology. References Cappellini, E., Welker, F., Pandolfi, L., Ramos-Madrigal, J., Samodova, D., Rüther, P. L., Fotakis, A. K., Lyon, D., Moreno-Mayar, J. V., Bukhsianidze, M., Rakownikow Jersie-Christensen, R., Mackie, M., Ginolhac, A., Ferring, R., Tappen, M., Palkopoulou, E., Dickinson, M. R., Stafford, T. W., Chan, Y. L., … Willerslev, E. (2019). Early Pleistocene enamel proteome from Dmanisi resolves Stephanorhinus phylogeny. Nature, 574(7776), 103–107. https://doi.org/10.1038/s41586-019-1555-y Douka, K., Brown, S., Higham, T., Pääbo, S., Derevianko, A., and Shunkov, M. (2019). FINDER project: Collagen fingerprinting (ZooMS) for the identification of new human fossils. Antiquity, 93(367), e1. https://doi.org/10.15184/aqy.2019.3 Lugli, F., Nava, A., Sorrentino, R., Vazzana, A., Bortolini, E., Oxilia, G., Silvestrini, S., Nannini, N., Bondioli, L., Fewlass, H., Talamo, S., Bard, E., Mancini, L., Müller, W., Romandini, M., and Benazzi, S. (2022). Tracing the mobility of a Late Epigravettian (~ 13 ka) male infant from Grotte di Pradis (Northeastern Italian Prealps) at high-temporal resolution. Scientific Reports, 12(1), 8104. https://doi.org/10.1038/s41598-022-12193-6 Ostrom, P. H., Schall, M., Gandhi, H., Shen, T.-L., Hauschka, P. V., Strahler, J. R., and Gage, D. A. (2000). New strategies for characterizing ancient proteins using matrix-assisted laser desorption ionization mass spectrometry. Geochimica et Cosmochimica Acta, 64(6), 1043–1050. https://doi.org/10.1016/S0016-7037(99)00381-6 Patramanis, I., Ramos-Madrigal, J., Cappellini, E., and Racimo, F. (2022). Hominid Palaeoproteomic Reference Dataset (1.0.1) [dataset]. Zenodo. https://doi.org/10.5281/ZENODO.7333226 Patramanis, I., Ramos-Madrigal, J., Cappellini, E., and Racimo, F. (2023). PaleoProPhyler: A reproducible pipeline for phylogenetic inference using ancient proteins. BioRxiv, 519721, ver. 3 peer-reviewed by PCI Paleo. https://doi.org/10.1101/2022.12.12.519721 Schroeter, E. R., Cleland, T. P., and Schweitzer, M. H. (2022). Deep Time Paleoproteomics: Looking Forward. Journal of Proteome Research, 21(1), 9–19. https://doi.org/10.1021/acs.jproteome.1c00755 Shapiro, B., Barlow, A., Heintzman, P. D., Hofreiter, M., Paijmans, J. L. A., and Soares, A. E. R. (Eds.). (2019). Ancient DNA: Methods and Protocols (2nd ed., Vol. 1963). Humana, New York. https://doi.org/10.1007/978-1-4939-9176-1 Warinner, C., Korzow Richter, K., and Collins, M. J. (2022). Paleoproteomics. Chemical Reviews, 122(16), 13401–13446. https://doi.org/10.1021/acs.chemrev.1c00703 Welker, F., Ramos-Madrigal, J., Gutenbrunner, P., Mackie, M., Tiwary, S., Rakownikow Jersie-Christensen, R., Chiva, C., Dickinson, M. R., Kuhlwilm, M., De Manuel, M., Gelabert, P., Martinón-Torres, M., Margvelashvili, A., Arsuaga, J. L., Carbonell, E., Marques-Bonet, T., Penkman, K., Sabidó, E., Cox, J., … Cappellini, E. (2020). The dental proteome of Homo antecessor. Nature, 580(7802), 235–238. https://doi.org/10.1038/s41586-020-2153-8 Welker, F., Ramos-Madrigal, J., Kuhlwilm, M., Liao, W., Gutenbrunner, P., De Manuel, M., Samodova, D., Mackie, M., Allentoft, M. E., Bacon, A.-M., Collins, M. J., Cox, J., Lalueza-Fox, C., Olsen, J. V., Demeter, F., Wang, W., Marques-Bonet, T., and Cappellini, E. (2019). Enamel proteome shows that Gigantopithecus was an early diverging pongine. Nature, 576(7786), 262–265. https://doi.org/10.1038/s41586-019-1728-8 | PaleoProPhyler: a reproducible pipeline for phylogenetic inference using ancient proteins | Ioannis Patramanis, Jazmín Ramos-Madrigal, Enrico Cappellini, Fernando Racimo | <p>Ancient proteins from fossilized or semi-fossilized remains can yield phylogenetic information at broad temporal horizons, in some cases even millions of years into the past. In recent years, peptides extracted from archaic hominins and long-ex... |  | Evolutionary biology, Paleoanthropology, Paleogenetics & Ancient DNA, Phylogenetics | Leslea Hlusko | 2023-02-24 13:40:12 | View | |
22 Sep 2018
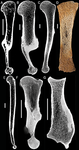
Palaeobiological inferences based on long bone epiphyseal and diaphyseal structure - the forelimb of xenarthrans (Mammalia)Eli Amson & John A. Nyakatura https://doi.org/10.1101/318121Inferences on the lifestyle of fossil xenarthrans based on limb long bone inner structureRecommended by Alexandra Houssaye based on reviews by Andrew Pitsillides and 1 anonymous reviewerBone inner structure bears a strong functional signal and can be used in paleontology to make inferences about the ecology of fossil forms. The increasing use of microtomography enables to analyze both cortical and trabecular features in three dimensions, and thus in long bones to investigate the diaphyseal and epiphyseal structures. Moreover, this can now be done through quantitative, and not only qualitative analyses. Studies focusing on the diaphyseal inner structure (cortical bone and sometimes also spongious bone) of long bones are rather numerous, but essentially based on 2D sections. It is only recently that analyses of the whole diaphyseal structure have been investigated. Studies on the trabecular architecture are much rarer. Amson & Nyakatura (2018) propose a comparative quantitative analysis combining parameters of the epiphyseal trabecular architecture and of the diaphyseal structure, using phylogenetically informed discriminant analyses, and with the aim of inferring the lifestyle of extinct taxa. The group of interest is xenarthrans, one of the four major extant clades of placental mammals. Xenarthrans exhibit different lifestyles, from fully terrestrial to arboreal, and show various degrees of fossoriality. The authors analyzed forelimb long bones of some fossil sloths and made comparisons with several species of extant xenarthrans. The aim was notably to discuss the degree of arboreality and fossoriality of these fossil forms. This study is among the first ones to conjointly analyze both diaphyseal and trabecular parameters to characterize lifestyles, and the first one outside of primates. No fossil form could undoubtedly be assigned to one lifestyle exhibited by extant xenarthrans, though some previous ecological hypotheses could be corroborated. This study also raised some technical challenges, linked to the sample and to the parameters studied, and thus constitutes a great step, from which to go further. References Amson, E., & Nyakatura, J. A. (2018). Palaeobiological inferences based on long bone epiphyseal and diaphyseal structure - the forelimb of xenarthrans (Mammalia). bioRxiv, 318121, ver. 5 peer-reviewed and recommended by PCI Paleo. doi: 10.1101/318121 | Palaeobiological inferences based on long bone epiphyseal and diaphyseal structure - the forelimb of xenarthrans (Mammalia) | Eli Amson & John A. Nyakatura | <p>Trabecular architecture (i.e., the main orientation of the bone trabeculae, their number, mean thickness, spacing, etc.) has been shown experimentally to adapt with great accuracy and sensitivity to the loadings applied to the bone during life.... |  | Biomechanics & Functional morphology, Comparative anatomy, Evolutionary biology, Histology, Methods, Morphological evolution, Paleobiology, Vertebrate paleontology | Alexandra Houssaye | 2018-05-14 08:35:20 | View | |
26 Oct 2023
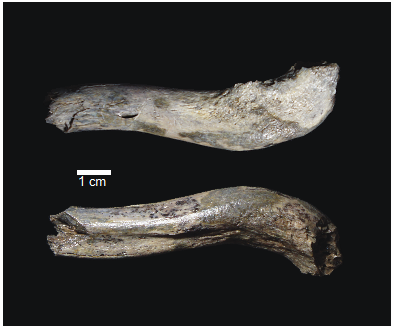
OH 89: A newly described ~1.8-million-year-old hominid clavicle from Olduvai GorgeCatherine E. Taylor, Fidelis Masao, Jackson K. Njau, Agustino Venance Songita, Leslea J. Hlusko https://doi.org/10.1101/2023.02.02.526656A new method for measuring clavicular curvatureRecommended by Nuria Garcia based on reviews by 2 anonymous reviewersThe evolution of the hominid clavicle has not been studied in depth by paleoanthropologists given its high morphological variability and the scarcity of complete diagnosable specimens. A nearly complete Nacholapithecus clavicle from Kenya (Senut et al. 2004) together with a fragment from Ardipithecus from the Afar region of Ethiopia (Lovejoy et al. 2009) complete our knowledge of the Miocene record. The Australopithecus collection of clavicles from Eastern and South African Plio-Pleistocene sites is slightly more abundant but mostly represented by fragmentary specimens. The number of fossil clavicles increases for the genus Homo from more recent sites and thus our potential knowledge about the shoulder evolution. In their new contribution, Taylor et al. (2023) present a detailed analysis of OH 89, a ~1.8-million-year-old partial hominin clavicle recovered from Olduvai Gorge (Tanzania). The work goes over previous studies which included clavicles found in the hominid fossil record. The text is accompanied by useful tables of data and a series of excellent photographs. It is a great opportunity to learn its role in the evolution of the hominid shoulder gird as clavicles are relatively poorly preserved in the fossil record compared to other long bones. The study compares the specimen OH 89 with five other hominid clavicles and a sample of 25 modern clavicles, 30 Gorilla, 31 Pan and 7 Papio. The authors propose a new methodology for measuring clavicular curvature using measurements of sternal and acromial curvature, from which an overall curvature measurement is calculated. The study of OH 89 provides good evidence about the hominid who lived 1.8 million years ago in the Olduvai Gorge region. This time period is especially relevant because it can help to understand the morphological changes that occurred between Australopithecus and the appearance of Homo. The authors conclude that OH 89 is the largest of the hominid clavicles included in the analysis. Although they are not able to assign this partial element to species level, this clavicle from Olduvai is at the larger end of the variation observed in Homo sapiens and show similarities to modern humans, especially when analysing the estimated sinusoidal curvature. References Lovejoy, C. O., Suwa, G., Simpson, S. W., Matternes, J. H., and White, T. D. (2009). The Great Divides: Ardipithecus ramidus peveals the postcrania of our last common ancestors with African apes. Science, 326(5949), 73–106. https://doi.org/10.1126/science.1175833 Senut, B., Nakatsukasa, M., Kunimatsu, Y., Nakano, Y., Takano, T., Tsujikawa, H., Shimizu, D., Kagaya, M., and Ishida, H. (2004). Preliminary analysis of Nacholapithecus scapula and clavicle from Nachola, Kenya. Primates, 45(2), 97–104. https://doi.org/10.1007/s10329-003-0073-5 Taylor, C., Masao, F., Njau, J. K., Songita, A. V., and Hlusko, L. J. (2023). OH 89: A newly described ∼1.8-million-year-old hominid clavicle from Olduvai Gorge. bioRxiv, 526656, ver. 6 peer-reviewed by PCI Paleo. https://doi.org/10.1101/2023.02.02.526656 | OH 89: A newly described ~1.8-million-year-old hominid clavicle from Olduvai Gorge | Catherine E. Taylor, Fidelis Masao, Jackson K. Njau, Agustino Venance Songita, Leslea J. Hlusko | <p>Objectives: Here, we describe the morphology and geologic context of OH 89, a ~1.8-million-year-old partial hominid clavicle from Olduvai Gorge, Tanzania. We compare the morphology and clavicular curvature of OH 89 to modern humans, extant apes... |  | Comparative anatomy, Evolutionary biology, Fossil record, Methods, Morphological evolution, Paleoanthropology, Vertebrate paleontology | Nuria Garcia | 2023-02-08 19:45:01 | View |
MANAGING BOARD
Jérémy Anquetin
Faysal Bibi
Guillaume Billet
Andrew A. Farke
Franck Guy
Leslea J. Hlusko
Melanie Hopkins
Cynthia V. Looy
Jesús Marugán-Lobón
Ilaria Mazzini
P. David Polly
Caroline A.E. Strömberg










