

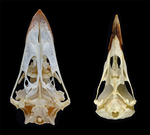
Today’s birds are divided into two deeply divergent and historically well-documented groups: Palaeognathae and Neognathae. Palaeognaths include both the flight-capable tinamous as well as the flightless ratites (ostriches, rheas, kiwis, cassowaries, and kin). Neognaths include all other modern birds, ranging from sparrows to penguins to hummingbirds. The clade names refer to the anatomy of the palate, with the “old jaws” (palaeognaths) originally thought to more closely resemble an ancestral reptilian condition and the “new jaws” (neognaths) showing a uniquely modified bony configuration. This particularly manifests in the pterygoid-palatine complex (PPC) in the palate, formed from pairs of pterygoids and palatines alongside a single midline vomer. In palaeognaths, the vomer is comparatively large and the pterygoid and palatine are relatively tightly connected. The PPC is more mobile in neognaths, with a variably shaped vomer, which is sometimes even absent. Although both groups of birds show cranial kinesis, neognaths exhibit a much more pronounced degree of kinesis versus palaeognaths, due in part to the tighter nature of the palaeognath pterygoid/palatine interfaces.
A previous paper (Hu et al. 2019) used 3D geometric morphometrics to compare the shape of the vomer across neognaths and palaeognaths. Among other findings, this work suggested that each clade had a distinct vomer morphology, with palaeognaths more similar to the ancestral condition (i.e., that of non-avian dinosaurs). This observation was extended to support inferences of limited vs. less limited cranial kinesis in various extinct species, based in part on observations of vomer shape. A new preprint by Plateau and Foth (2021) presents a reanalysis of Hu et al.’s data, specifically focusing on allometric effects. In short, the new analysis looks at how size correlates (or doesn't correlate) with vomer shape.
Plateau and Foth (2021) found that when size effects are included, differences between palaeognaths and neognaths are less than the “raw” (uncorrected) shape data suggest. It is much harder to tell bird groups apart! Certainly, there are still some general differences, but some separations in morphospace close up when allometry—the interrelationship between shape and size—is considered. Plateau and Foth (2021) use this finding to suggest that 1) vomer shape alone is not a completely reliable proxy for inferring the phylogenetic affinities of a particular bird; and 2) the vomer is only one small component of the cranial kinetic system, and thus its shape is of limited utility for inferring cranial kinesis capabilities when considered independently from the rest of the relevant skull bones.
References
Hu, H., Sansalone, G., Wroe, S., McDonald, P. G., O’Connor, J. K., Li, Z., Xu, X., & Zhou, Z. (2019). Evolution of the vomer and its implications for cranial kinesis in Paraves. Proceedings of the National Academy of Sciences, 116(39), 19571–19578. doi: 10.1073/pnas.1907754116
Plateau, O., & Foth, C. (2021). The impact of allometry on vomer shape and its implications for the taxonomy and cranial kinesis of crown-group birds. BioRxiv, 184101, ver. 3 peer-reviewed by PCI Paleo. doi: 10.1101/2020.07.02.184101
DOI or URL of the preprint: https://doi.org/10.1101/2020.07.02.184101
Dear Dr. Farke,
Please find attached the revised version of our manuscript entitled “The impact of allometry on vomer shape and its implications for the taxonomy and cranial kinesis of crown-group birds”. Both reviewers provided useful comments, and we are happy to acknowledge that we included most of their suggestions to the new version. In the few cases where we did not agree with the reviewer, we provided a detailed response. All responses to the reviewers are highlighted in bold.
We hope that you are satisfied with the revised version and looking forward to hear from you.
With kind regards,
Christian Foth (on behalf of all authors)
Present study lead by Plateau & Foth and titled ‘The impact of allometry on vomer shape and its implications for the taxonomy and cranial kinesis of crown-group birds’ deeply addresses a relevant aspect that strongly affect the morphological evolution of several anatomical structures: size. The approach you propose is essential to correctly understand how the influence of this central structure is in craniofacial and PPC configuration, and also in kinesis performance among other cranial traits. This is important specially when such structures are suggested as taxonomic distinguishing. Thus, it is important that you emphasize that, although there are important morphological differences between the main groups of modern birds, these are mostly due to (or related to) the evolution of body size, a fundamental biological trait, and not so much as shape changes themselves (i.e. vomer morphology is not independent, in this case from its size, and if you don’t take raw size differences as a taxonomic trait, you shouldn’t take as such a morphological trait associated with it).
The authors address this important question with a wide and dense combination of methods which look for the pure morphological differences that are not under the widespread influence of size, but I suggest below some commonplace aspects that I think they could contribute to the robustness of the study and make it more complete.
I would suggest to estimates the morphological disparity from the Procrustes shape variables for each group and compares it between groups with pairwise comparisons. Thus, you can compare statistically the morphological variation for each group and their distinct distribution across the morphospace, especially when you extract size and groups collapse, obtaining different significant results in size-affected shapes.
Also, another option to provide robustness to the hypothesis that vomer’s morphology is not a good taxonomic character is estimating the phylogenetic signal both in shape variables, centroid size and centroid size proportional to skull size. You should obtain empirical K values lower to the predicted value of K = 1 for a Brownian Motion model of evolution indicating weaker phylogenetic signal and a higher degree of convergence. Additionally, it should be observed a similar distribution across the phylogeny between shape variables and size variables plotting them as continuous variables.
I would suggest to carry out the allometric regression with Phylogenetic Generalized Least Squares and group by group, running pairwise comparisons between their slopes in order to know how is the allometric influence. Allometry seems to affect specially in Paleognathae considering that when you extract size the difficulty to distinguish these birds from the rest increases. This aspect could be due to ratites reaches bigger sizes.
Finally, you discuss about kinesis and why is not be possible that its different types are related to vomer shape. In order to know this lack of correlation I would recommend grouping the sample by kinesis type, estimating morphological disparities for each group and comparing statistically their distribution across the morphospace. It should collapse widely. Also, it would be interesting to explore by PGLS how different types of kinesis are related to different allometric scaling, because it could be that the shapesize relationships from distinct kinesis types are similar and not significantly different. Furthermore, to do that I would take into account as an important aspect for kinesis performance the vomer size relative to skull size for a better addressing of their significance on such trait.
For the figure 1 I suggest to add a close up of the region that anatomically distinguishes paleognathans from neognathans and a better schedule that could show the stiffness or mobility of the PPC (for example, marking or highlighting the structures that characterize each type of PPC).
For the PCAs (figure 2) I would suggest trying to plot vomer size and vomer size overall skull size as dot size. Thus, it could be visualized the polarization of size on the morphospace and the wide divergence between paleognathans and neognathans. Finally, the interpretation of the graphics would improve if you add silhouettes representing different groups and if you mark above from which analysis they come or if they are taking size into account or not.
This is an interesting piece of work, and takes further the previous work of Hu et al. (2019), of which a reanalysis of whose data it basically consists. It is a necessary further analysis of the data, and it appears to be sound methodologically and in its basic conclusions. I am not a particular expert in the groups at hand however, and thus my conclusions regarding its soundness are not based on specific knowledge of the taxa.
One major query I have, given the very specific nature of the work, and the fact that it basically consists of a reanalysis of a recent dataset, is perhaps it would be better published in PNAS as a reply to the original paper of Hu et al.? This would give the authors a chance to respond to the comments, and mean it was read by the scientific public as a direct continuation of the original work. …So yes, perhaps this could be suggested to the authors, if they have not already considered it?
My only other major comment really, given that the “meat” of the work is pretty sound, is that the discussion ends rather abruptly, and doesn’t expand on the wider implications of the reanalysis of the data of Hu and colleagues. It would might be nice to have a concluding paragraph or two at the end of the discussion, with a bit of contextualisation of the study’s findings in terms of their broader implications for avian evolution and evolutionary processes and analysis more broadly. Any main points here could also be added as a sentence of the abstract. …Specifically, the wider implication discussed by Hu et al. for the radiation of the avian crown group should be addressed, and what differences the reanalysis makes for these conclusions outlined.
Some other ideas of wider implications: isolated elements may not reliably group taxa, and can be more strongly subject to convergence; the avian skull has seen a labile pattern of evolution, with repeated development of similar morphology – perhaps this is broadly the case in many groups?; it should be checked that allometry is not the driving cause behind seemingly “primitive” shapes and characteristics etc. etc. Also, can you place the vomer development in birds somehow in the wider context of archosaur evolution, for example of the feeding and sensory systems? There are a few minor linguistic points (mostly just formal v. informal issues, or clarity), which I annotated in the PDF.
In the reference list, the book references seem a bit lacking in details (e.g. number of pages, editors) – if this is acceptable for PCI Palaeo that’s OK (ask the editor!), but it is also not consistent throughout currently.
In figure captions 2, several abbreviations (e.g. PCA) are used without being defined. I think they should be defined the first time used in each figure caption so that they can be read without referring to the text.
I would recommend adding silhouettes of either whole animals or at least skulls on figures 2 and 3, to make it clearer what is what. The colours are great, but silhouettes would help a lot.
There are also some issues recurring throughout the manuscript with taxon names. These have been annotated the first few times they occur in the PDF, but should be checked throughout:
- The abbreviation of genus names is not consistently done (sometimes not abbreviated) and is confusing. This also goes for figure 1. “S.” is used in rapid succession for three different genera! I would recommend you state the full genus name in all cases, and if you want to abbreviate then do so by removing the species name in monospecific taxa. ...If you really want to abbreviate genera, can you reduce them by only enough letters so no abbreviations overlap, for example "St." for "Struthio", "Si" for "Sinovenator" etc.?
- The capitalization of Latin versus anglified clade names does not seem to be always correct. Original Latin (e.g. “Inopinaves”) should be capitalized, and anglified versions (I presume in this case it would be “inopinavians”) should not. If in doubt, I’d say stick to the Latin and capitalize.
- I guess “aequorlitornithines” is maybe the correct anglification of “Aequorlitornithes”, but the only place I found it used so far was this preprint! (I guess it’s only been in existence since 2015, so that’s why) Maybe stick to the capitalized Latin? I am not an expert in avian taxonomy though, so your call.
- Sometimes “Galloanseres” or “galloanserinae” have been used
-“jackknife” isn’t hyphenated!
Thank you for your extreme patience and understanding during this protracted review process. I have now received two very constructive reviews, with some suggestions to address should you choose to revise your preprint. These are (hopefully) relatively straight-forward to incorporate, and in my opinion are equivalent to a request for minor revisions. As one additional note to the reviewer comments, please adjust colors and symbols In Figure 2 and Figure 3, to improve accessibility for those who are color-blind. The reviewer suggestion of including some silhouettes is optional, but makes sense. If you do add silhouettes, please make sure that they can be used under an appropriate public domain or Creative Commons Attribution (CC-BY) license.
Present study lead by Plateau & Foth and titled ‘The impact of allometry on vomer shape and its implications for the taxonomy and cranial kinesis of crown-group birds’ deeply addresses a relevant aspect that strongly affect the morphological evolution of several anatomical structures: size. The approach you propose is essential to correctly understand how the influence of this central structure is in craniofacial and PPC configuration, and also in kinesis performance among other cranial traits. This is important specially when such structures are suggested as taxonomic distinguishing. Thus, it is important that you emphasize that, although there are important morphological differences between the main groups of modern birds, these are mostly due to (or related to) the evolution of body size, a fundamental biological trait, and not so much as shape changes themselves (i.e. vomer morphology is not independent, in this case from its size, and if you don’t take raw size differences as a taxonomic trait, you shouldn’t take as such a morphological trait associated with it).
The authors address this important question with a wide and dense combination of methods which look for the pure morphological differences that are not under the widespread influence of size, but I suggest below some commonplace aspects that I think they could contribute to the robustness of the study and make it more complete.
I would suggest to estimates the morphological disparity from the Procrustes shape variables for each group and compares it between groups with pairwise comparisons. Thus, you can compare statistically the morphological variation for each group and their distinct distribution across the morphospace, especially when you extract size and groups collapse, obtaining different significant results in sizeaffected shapes.
Also, another option to provide robustness to the hypothesis that vomer’s morphology is not a good taxonomic character is estimating the phylogenetic signal both in shape variables, centroid size and centroid size proportional to skull size. You should obtain empirical K values lower to the predicted value of K = 1 for a Brownian Motion model of evolution indicating weaker phylogenetic signal and a higher degree of convergence. Additionally, it should be observed a similar distribution across the phylogeny between shape variables and size variables plotting them as continuous variables.
I would suggest to carry out the allometric regression with Phylogenetic Generalized Least Squares and group by group, running pairwise comparisons between their slopes in order to know how is the allometric influence. Allometry seems to affect specially in Paleognathae considering that when you extract size the difficulty to distinguish these birds from the rest increases. This aspect could be due to ratites reaches bigger sizes.
Finally, you discuss about kinesis and why is not be possible that its different types are related to vomer shape. In order to know this lack of correlation I would recommend grouping the sample by kinesis type, estimating morphological disparities for each group and comparing statistically their distribution across the morphospace. It should collapse widely. Also, it would be interesting to explore by PGLS how different types of kinesis are related to different allometric scaling, because it could be that the shapesize relationships from distinct kinesis types are similar and not significantly different. Furthermore, to do that I would take into account as an important aspect for kinesis performance the vomer size relative to skull size for a better addressing of their significance on such trait.
For the figure 1 I suggest to add a close up of the region that anatomically distinguishes paleognathans from neognathans and a better schedule that could show the stiffness or mobility of the PPC (for example, marking or highlighting the structures that characterize each type of PPC).
For the PCAs (figure 2) I would suggest trying to plot vomer size and vomer size overall skull size as dot size. Thus, it could be visualized the polarization of size on the morphospace and the wide divergence between paleognathans and neognathans. Finally, the interpretation of the graphics would improve if you add silhouettes representing different groups and if you mark above from which analysis they come or if they are taking size into account or not.
There are no more comments that both authors and editors must take into account for the review of the manuscript.
Download the review https://doi.org/10.24072/pci.paleo.100013.rev11 , 23 Nov 2020
, 23 Nov 2020This is an interesting piece of work, and takes further the previous work of Hu et al. (2019), of which a reanalysis of whose data it basically consists. It is a necessary further analysis of the data, and it appears to be sound methodologically and in its basic conclusions. I am not a particular expert in the groups at hand however, and thus my conclusions regarding its soundness are not based on specific knowledge of the taxa.
One major query I have, given the very specific nature of the work, and the fact that it basically consists of a reanalysis of a recent dataset, is perhaps it would be better published in PNAS as a reply to the original paper of Hu et al.? This would give the authors a chance to respond to the comments, and mean it was read by the scientific public as a direct continuation of the original work. …So yes, perhaps this could be suggested to the authors, if they have not already considered it?
My only other major comment really, given that the “meat” of the work is pretty sound, is that the discussion ends rather abruptly, and doesn’t expand on the wider implications of the reanalysis of the data of Hu and colleagues. It would might be nice to have a concluding paragraph or two at the end of the discussion, with a bit of contextualisation of the study’s findings in terms of their broader implications for avian evolution and evolutionary processes and analysis more broadly. Any main points here could also be added as a sentence of the abstract. …Specifically, the wider implication discussed by Hu et al. for the radiation of the avian crown group should be addressed, and what differences the reanalysis makes for these conclusions outlined.
Some other ideas of wider implications: isolated elements may not reliably group taxa, and can be more strongly subject to convergence; the avian skull has seen a labile pattern of evolution, with repeated development of similar morphology – perhaps this is broadly the case in many groups?; it should be checked that allometry is not the driving cause behind seemingly “primitive” shapes and characteristics etc. etc. Also, can you place the vomer development in birds somehow in the wider context of archosaur evolution, for example of the feeding and sensory systems? There are a few minor linguistic points (mostly just formal v. informal issues, or clarity), which I annotated in the PDF.
In the reference list, the book references seem a bit lacking in details (e.g. number of pages, editors) – if this is acceptable for PCI Palaeo that’s OK (ask the editor!), but it is also not consistent throughout currently.
In figure captions 2, several abbreviations (e.g. PCA) are used without being defined. I think they should be defined the first time used in each figure caption so that they can be read without referring to the text.
I would recommend adding silhouettes of either whole animals or at least skulls on figures 2 and 3, to make it clearer what is what. The colours are great, but silhouettes would help a lot.
There are also some issues recurring throughout the manuscript with taxon names. These have been annotated the first few times they occur in the PDF, but should be checked throughout:
-The abbreviation of genus names is not consistently done (sometimes not abbreviated) and is confusing. This also goes for figure 1. “S.” is used in rapid succession for three different genera! I would recommend you state the full genus name in all cases, and if you want to abbreviate then do so by removing the species name in monospecific taxa. ...If you really want to abbreviate genera, can you reduce them by only enough letters so no abbreviations overlap, for example "St." for "Struthio", "Si" for "Sinovenator" etc.?
-The capitalization of Latin versus anglified clade names does not seem to be always correct. Original Latin (e.g. “Inopinaves”) should be capitalized, and anglified versions (I presume in this case it would be “inopinavians”) should not. If in doubt, I’d say stick to the Latin and capitalize.
-I guess “aequorlitornithines” is maybe the correct anglification of “Aequorlitornithes”, but the only place I found it used so far was this preprint! (I guess it’s only been in existence since 2015, so that’s why) Maybe stick to the capitalized Latin? I am not an expert in avian taxonomy though, so your call.
-Sometimes “Galloanseres” or “galloanserinae” have been used
-“jackknife” isn’t hyphenated!
Download the review https://doi.org/10.24072/pci.paleo.100013.rev12