Latest recommendations

| Id | Title▼ | Authors | Abstract | Picture | Thematic fields | Recommender | Reviewers | Submission date | |
|---|---|---|---|---|---|---|---|---|---|
26 Apr 2024
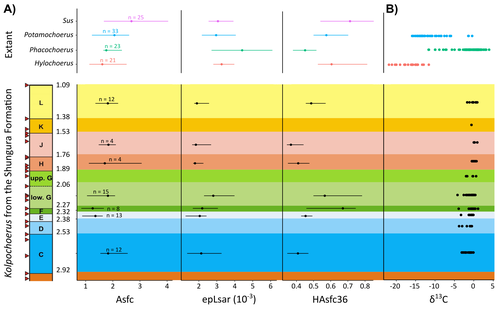
New insights on feeding habits of Kolpochoerus from the Shungura Formation (Lower Omo Valley, Ethiopia) using dental microwear texture analysisMargot Louail, Antoine Souron, Gildas Merceron, Jean-Renaud Boisserie https://osf.io/preprints/paleorxiv/dbgtpDental microwear texture analysis of suid teeth from the Shungura Formation of the Omo Valley, EthiopiaRecommended by Denise Su based on reviews by Daniela E. Winkler and Kari PrassackSuidae are well-represented in Plio-Pleistocene African hominin sites and are particularly important for biochronological assessments. Their ubiquity in hominin sites combined with multiple appearances of what appears to be graminivorous adaptations in the lineage (Harris & White, 1979) suggest that they have the potential to contribute to our understanding of Plio-Pleistocene paleoenvironments. While they have been generally understudied in this respect, there has been recent focus on their diets to understand the paleoenvironments of early hominin habitats. Of particular interest is Kolpochoerus, one of the most abundant suid genera in the Plio-Pleistocene with a wide geographic distribution and diverse dental morphologies (Harris & White, 1979). In this study, Louail et al. (2024) present the results of the first dental microwear texture analysis (DMTA) conducted on suids from the Shungura Formation of the Omo Valley, an important Plio-Pleistocene hominin site that records an almost continuous sedimentary record from ca. 3.75 Ma to 1.0 Ma (Heinzelin 1983; McDougall et al., 2012; Kidane et al., 2014). Dental microwear is one of the main proxies in understanding diet in fossil mammals, particularly herbivores, and DMTA has been shown to be effective in differentiating inter- and intra-species dietary differences (e.g., Scott et al., 2006; 2012; Merceron et al., 2010). However, only a few studies have applied this method to extinct suids (Souron et al., 2015; Ungar et al., 2020), making this study especially pertinent for those interested in suid dietary evolution or hominin paleoecology. In addition to examining DMT variations of Kolpochoerus specimens from Omo, Louail et al. (2024) also expanded the modern comparative data set to include larger samples of African suids with different diets from herbivores to omnivores to better interpret the fossil data. They found that DMTA distinguishes between extant suid taxa, reflecting differences in diet, indicating that DMT can be used to examine the diets of fossil suids. The results suggest that Kolpochoerus at Omo had a substantially different diet from any extant suid taxon and that although its anistropy values increased through time, they remain well below those observed in modern Phacochoerus that specializes in fibrous, abrasive plants. Based on these results, in combination with comparative and experimental DMT, enamel carbon isotopic, and morphological data, Louail et al. (2024) propose that Omo Kolpochoerus preferred short, soft and low abrasive herbaceous plants (e.g., fresh grass shoots), probably in more mesic habitats. Louail et al. (2024) note that with the wide temporal and geographic distribution of Kolpochoerus, different species and populations may have had different feeding habits as they exploited different local habitats. However, it is noteworthy that similar inferences were made at other hominin sites based on other types of dietary data (e.g., Harris & Cerling, 2002; Rannikko et al., 2017, 2020; Yang et al., 2022). If this is an indication of their habitat preferences, the wide-ranging distribution of Kolpochoerus may suggest that mesic habitats with short, soft herbaceous plants were present in various proportions at most Plio-Pleistocene hominin sites. References Harris, J. M., and Cerling, T. E. (2002). Dietary adaptations of extant and Neogene African suids. Journal of Zoology, 256(1), 45–54. https://doi.org/10.1017/S0952836902000067 Harris, J. M., and White, T. D. (1979). Evolution of the Plio-Pleistocene African Suidae. Transactions of the American Philosophical Society, 69(2), 1–128. https://doi.org/10.2307/1006288 Heinzelin, J. de. (1983). The Omo Group. Archives of the International Omo Research Expedition. Volume 85. Annales du Musée Royal de l’Afrique Centrale, série 8, Sciences géologiques, Tervuren, 388 p. Kidane, T., Brown, F. H., and Kidney, C. (2014). Magnetostratigraphy of the fossil-rich Shungura Formation, southwest Ethiopia. Journal of African Earth Sciences, 97, 207–223. https://doi.org/10.1016/j.jafrearsci.2014.05.005 Louail, M., Souron, A., Merceron, G., and Boisserie, J.-R. (2024). New insights on feeding habits of Kolpochoerus from the Shungura Formation (Lower Omo Valley, Ethiopia) using dental microwear texture analysis. PaleorXiv, dbgtp, ver. 3, peer-reviewed by PCI Paleo. https://doi.org/10.31233/osf.io/dbgtp McDougall, I., Brown, F. H., Vasconcelos, P. M., Cohen, B. E., Thiede, D. S., and Buchanan, M. J. (2012). New single crystal 40Ar/39Ar ages improve time scale for deposition of the Omo Group, Omo–Turkana Basin, East Africa. Journal of the Geological Society, 169(2), 213–226. https://doi.org/10.1144/0016-76492010-188 Merceron, G., Escarguel, G., Angibault, J.-M., and Verheyden-Tixier, H. (2010). Can dental microwear textures record inter-individual dietary variations? PLoS ONE, 5(3), e9542. https://doi.org/10.1371/journal.pone.0009542 Rannikko, J., Adhikari, H., Karme, A., Žliobaitė, I., and Fortelius, M. (2020). The case of the grass‐eating suids in the Plio‐Pleistocene Turkana Basin: 3D dental topography in relation to diet in extant and fossil pigs. Journal of Morphology, 281(3), 348–364. https://doi.org/10.1002/jmor.21103 Rannikko, J., Žliobaitė, I., and Fortelius, M. (2017). Relative abundances and palaeoecology of four suid genera in the Turkana Basin, Kenya, during the late Miocene to Pleistocene. Palaeogeography, Palaeoclimatology, Palaeoecology, 487, 187–193. https://doi.org/10.1016/j.palaeo.2017.08.033 Scott, R. S., Teaford, M. F., and Ungar, P. S. (2012). Dental microwear texture and anthropoid diets. American Journal of Physical Anthropology, 147(4), 551–579. https://doi.org/10.1002/ajpa.22007 Scott, R. S., Ungar, P. S., Bergstrom, T. S., Brown, C. A., Childs, B. E., Teaford, M. F., and Walker, A. (2006). Dental microwear texture analysis: Technical considerations. Journal of Human Evolution, 51(4), 339–349. https://doi.org/10.1016/j.jhevol.2006.04.006 Souron, A., Merceron, G., Blondel, C., Brunetière, N., Colyn, M., Hofman-Kamińska, E., and Boisserie, J.-R. (2015). Three-dimensional dental microwear texture analysis and diet in extant Suidae (Mammalia: Cetartiodactyla). Mammalia, 79(3). https://doi.org/10.1515/mammalia-2014-0023 Ungar, P. S., Abella, E. F., Burgman, J. H. E., Lazagabaster, I. A., Scott, J. R., Delezene, L. K., Manthi, F. K., Plavcan, J. M., and Ward, C. V. (2020). Dental microwear and Pliocene paleocommunity ecology of bovids, primates, rodents, and suids at Kanapoi. Journal of Human Evolution, 140, 102315. https://doi.org/10.1016/j.jhevol.2017.03.005 Yang, D., Pisano, A., Kolasa, J., Jashashvili, T., Kibii, J., Gomez Cano, A. R., Viriot, L., Grine, F. E., and Souron, A. (2022). Why the long teeth? Morphometric analysis suggests different selective pressures on functional occlusal traits in Plio-Pleistocene African suids. Paleobiology, 48(4), 655–676. https://doi.org/10.1017/pab.2022.11 | New insights on feeding habits of *Kolpochoerus* from the Shungura Formation (Lower Omo Valley, Ethiopia) using dental microwear texture analysis | Margot Louail, Antoine Souron, Gildas Merceron, Jean-Renaud Boisserie | <p>During the Neogene and the Quaternary, African suids show dental morphological changes considered to reflect adaptations to increasing specialization on graminivorous diets, notably in the genus <em>Kolpochoerus</em>. They tend to exhibit elong... |  | Paleoecology, Vertebrate paleontology | Denise Su | 2023-08-28 10:38:33 | View | |
25 Oct 2022
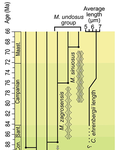
Morphometric changes in two Late Cretaceous calcareous nannofossil lineages support diversification fueled by long-term climate changeMohammad Javad Razmjooei, Nicolas Thibault https://doi.org/10.31233/osf.io/nfyc9Insights into mechanisms of coccolithophore speciation: How useful is cell size in delineating species?Recommended by Emilia Jarochowska based on reviews by Andrej Spiridonov and 1 anonymous reviewer based on reviews by Andrej Spiridonov and 1 anonymous reviewer
Calcareous plankton gives us perhaps the most complete record of microevolutionary changes in the fossil record (e.g. Tong et al., 2018; Weinkauf et al., 2019), but this opportunity is not exploited enough, as it requires meticulous work in documenting assemblage-level variation through time. Especially in organisms such as coccolithophores, understanding the meaning of secular trends in morphology warrants an understanding of the functional biology and ecology of these organisms. Razmjooei and Thibault (2022) achieve this in their painstaking analysis of two coccolithophore lineages, Cribrosphaerella ehrenbergii and Microrhabdulus, in the Late Cretaceous of Iran. They propose two episodes of morphological change. The first one, starting around 76 Ma in the late Campanian, is marked by a sudden shift towards larger sizes of C. ehrenbergii and the appearance of a new species M. zagrosensis from M. undulatus. The second episode around 69 Ma (Maastrichtian) is inferred from a gradual size increase and morphological changes leading to probably anagenetic speciation of M. sinuosus n.sp. The study remarkably analyzed the entire distributions of coccolith length and rod width, rather than focusing on summary statistics (De Baets et al., in press). This is important, because the range of variation determines the taxon’s evolvability with respect to the considered trait (Love et al., 2022). As the authors discuss, cell size in photosymbiotic unicellular organisms is not subject to the same constraints that will be familiar to researchers working e.g. on mammals (Niklas, 1994; Payne et al., 2009; Smith et al., 2016). Furthermore, temporal changes in size alone cannot be interpreted as evolutionary without knowledge of phenotypic plasticity and environmental clines present in the basin (Aloisi, 2015). The more important is that this study cross-tests size changes with other morphological parameters to examine whether their covariation supports inferred speciation events. The article addresses as well the effects of varying sedimentation rates (Hohmann, 2021) by, somewhat implicitly, correcting for the stratophenetic trend using an age-depth model and accounting for a hiatus. Such multifaceted approach as applied in this work is fundamental to unlock the dynamics of speciation offered by the microfossil record. The study highlights also the link between shifts in size and diversity. Klug et al. (2015) have previously demonstrated that these two variables are related, as higher diversity is more likely to lead to extreme values of morphological traits, but this study suggests that the relationship is more intertwined: environmentally-driven rise in morphological variability (and thus in size) can lead to diversification. It is a fantastic illustration of the complexity of morphological evolution that, if it can be evaluated in terms of mechanisms, provides an insight into the dynamics of speciation.
References Aloisi, G. (2015). Covariation of metabolic rates and cell size in coccolithophores. Biogeosciences, 12(15), 4665–4692. doi: 10.5194/bg-12-4665-2015 De Baets, K., Jarochowska, E., Buchwald, S. Z., Klug, C., and Korn, D. (In Press). Lithology controls ammonoid size distribution. Palaios. Hohmann, N. (2021). Incorporating information on varying sedimentation rates into palaeontological analyses. PALAIOS, 36(2), 53–67. doi: 10.2110/palo.2020.038 Klug, C., De Baets, K., Kröger, B., Bell, M. A., Korn, D., and Payne, J. L. (2015). Normal giants? Temporal and latitudinal shifts of Palaeozoic marine invertebrate gigantism and global change. Lethaia, 48(2), 267–288. doi: 10.1111/let.12104 Love, A. C., Grabowski, M., Houle, D., Liow, L. H., Porto, A., Tsuboi, M., Voje, K.L., and Hunt, G. (2022). Evolvability in the fossil record. Paleobiology, 48(2), 186–209. doi: 10.1017/pab.2021.36 Niklas, K. J. (1994). Plant allometry: The scaling of form and process. Chicago: University of Chicago Press. Payne, J. L., Boyer, A. G., Brown, J. H., Finnegan, S., Kowalewski, M., Krause, R. A., Lyons, S.K., McClain, C.R., McShea, D.W., Novack-Gottshall, P.M., Smith, F.A., Stempien, J.A., and Wang, S. C. (2009). Two-phase increase in the maximum size of life over 3.5 billion years reflects biological innovation and environmental opportunity. Proceedings of the National Academy of Sciences, 106(1), 24–27. doi: 10.1073/pnas.0806314106 Razmjooei, M. J., and Thibault, N. (2022). Morphometric changes in two Late Cretaceous calcareous nannofossil lineages support diversification fueled by long-term climate change. PaleorXiv, nfyc9, ver. 4, peer-reviewed by PCI Paleo. doi: 10.31233/osf.io/nfyc9 Smith, F. A., Payne, J. L., Heim, N. A., Balk, M. A., Finnegan, S., Kowalewski, M., Lyons, S.K., McClain, C.R., McShea, D.W., Novack-Gottshall, P.M., Anich, P.S., and Wang, S. C. (2016). Body size evolution across the Geozoic. Annual Review of Earth and Planetary Sciences, 44(1), 523–553. doi: 10.1146/annurev-earth-060115-012147 Tong, S., Gao, K., and Hutchins, D. A. (2018). Adaptive evolution in the coccolithophore Gephyrocapsa oceanica following 1,000 generations of selection under elevated CO2. Global Change Biology, 24(7), 3055–3064. doi: 10.1111/gcb.14065 Weinkauf, M. F. G., Bonitz, F. G. W., Martini, R., and Kučera, M. (2019). An extinction event in planktonic Foraminifera preceded by stabilizing selection. PLOS ONE, 14(10), e0223490. doi: 10.1371/journal.pone.0223490 | Morphometric changes in two Late Cretaceous calcareous nannofossil lineages support diversification fueled by long-term climate change | Mohammad Javad Razmjooei, Nicolas Thibault | <p>Morphometric changes have been investigated in the two groups of calcareous nannofossils, <em>Cribrosphaerella ehrenbergii</em> and <em>Microrhabdulus undosus</em> across the Campanian to Maastrichtian of the Zagros Basin of Iran. Results revea... |  | Biostratigraphy, Evolutionary theory, Fossil record, Microfossils, Micropaleontology, Morphological evolution, Morphometrics, Nanofossils, Paleobiodiversity, Paleobiology, Paleoceanography, Paleoclimatology, Paleoecology, Paleoenvironments, Taxonomy | Emilia Jarochowska | 2020-08-29 12:23:51 | View | |
20 Oct 2020
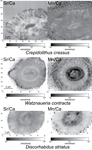
Evidence of high Sr/Ca in a Middle Jurassic murolith coccolith speciesBaptiste Suchéras-Marx, Fabienne Giraud, Alexandre Simionovici, Rémi Tucoulou, Isabelle Daniel https://doi.org/10.31233/osf.io/dcfuqNew results and challenges in Sr/Ca studies on Jurassic coccolithophoridsRecommended by Antonino Briguglio based on reviews by Kenneth De Baets and 1 anonymous reviewerThis interesting publication by Suchéras-Marx et al. (2020) highlights peculiar aspects of geochemistry in nannofossils, specifically coccolithophorids. One of the main application of geochemistry on fossil shells is to get hints on the physiology of such extinct taxa. Here, the authors try to get information on the calcification mechanism and processes in Jurassic coccoliths. Coccoliths build a test made of calcium carbonate and one of the most common geochemical proxies used for this fossil group is the Sr/Ca ratio. This isotopic ratio has good chances to be successfully used as a robust proxy for paleoenvironmental reconstruction, but, concerning Jurassic coccoliths things seem to be not straightforward. The authors managed to compare the isotopic value of Sr/Ca measured on Jurassic coccoliths from different taxonomic groups: the murolith Crepidolithus crassus and the placoliths Watznaueria contracta and Discorhabdus striatus. The results they got clearly show that the Sr/Ca ratio cannot be used as a universal proxy because these species exhibit very different values despite coming from the same stratigraphic level and having undergone minimal diagenetic modification. Data seem to point to a Sr/Ca ratio up to 10 times higher in the murolith species than in the placolith taxa (Suchéras-Marx et al., 2020). One of the explanation given here takes advantage of modern coccolith data and hints to specific polysaccharides that would control the growth of the long R unit in the murolith species. As always, there is plenty of space for additional research, possibly on modern taxa, to sort out the scientific questions that arise from this work. References Suchéras-Marx, B., Giraud, F., Simionovici, A., Tucoulou, R., & Daniel, I. (2020). Evidence of high Sr/Ca in a Middle Jurassic murolith coccolith species. PaleorXiv, dcfuq, version 7, peer-reviewed by PCI Paleo. doi: 10.31233/osf.io/dcfuq | Evidence of high Sr/Ca in a Middle Jurassic murolith coccolith species | Baptiste Suchéras-Marx, Fabienne Giraud, Alexandre Simionovici, Rémi Tucoulou, Isabelle Daniel | <p>Paleoceanographical reconstructions are often based on microfossil geochemical analyses. Coccoliths are the most ancient, abundant and continuous record of pelagic photic zone calcite producer organisms. Hence, they could be valuable substrates... |  | Microfossils, Micropaleontology, Nanofossils | Antonino Briguglio | 2020-05-18 16:11:35 | View | |
26 Mar 2024
Calibrations without raw data - a response to "Seasonal calibration of the end-cretaceous Chicxulub impact event"Melanie A.D. During, Dennis F.A.E. Voeten, Per E. Ahlberg https://osf.io/fu7rp/Questioning isotopic data from the end-CretaceousRecommended by Christina Belanger based on reviews by Thomas Cullen and 1 anonymous reviewerBeing able to follow the evidence and verify results is critical if we are to be confident in the findings of a scientific study. Here, During et al. (2024) comment on DePalma et al. (2021) and provide a detailed critique of the figures and methods presented that caused them to question the veracity of the isotopic data used to support a spring-time Chicxulub impact at the end-Cretaceous. Given DePalma et al. (2021) did not include a supplemental file containing the original isotopic data, the suspicions rose to accusations of data fabrication (Price, 2022). Subsequent investigations led by DePalma’s current academic institution, The University of Manchester, concluded that the study contained instances of poor research practice that constitute research misconduct, but did not find evidence of fabrication (Price, 2023). Importantly, the overall conclusions of DePalma et al. (2021) are not questioned and both the DePalma et al. (2021) study and a study by During et al. (2022) found that the end-Cretaceous impact occurred in spring. During et al. (2024) also propose some best practices for reporting isotopic data that can help future authors make sure the evidence underlying their conclusions are well documented. Some of these suggestions are commonly reflected in the methods sections of papers working with similar data, but they are not universally required of authors to report. Authors, research mentors, reviewers, and editors, may find this a useful set of guidelines that will help instill confidence in the science that is published. References DePalma, R. A., Oleinik, A. A., Gurche, L. P., Burnham, D. A., Klingler, J. J., McKinney, C. J., Cichocki, F. P., Larson, P. L., Egerton, V. M., Wogelius, R. A., Edwards, N. P., Bergmann, U., and Manning, P. L. (2021). Seasonal calibration of the end-cretaceous Chicxulub impact event. Scientific Reports, 11(1), 23704. https://doi.org/10.1038/s41598-021-03232-9 During, M. A. D., Smit, J., Voeten, D. F. A. E., Berruyer, C., Tafforeau, P., Sanchez, S., Stein, K. H. W., Verdegaal-Warmerdam, S. J. A., and Van Der Lubbe, J. H. J. L. (2022). The Mesozoic terminated in boreal spring. Nature, 603(7899), 91–94. https://doi.org/10.1038/s41586-022-04446-1 During, M. A. D., Voeten, D. F. A. E., and Ahlberg, P. E. (2024). Calibrations without raw data—A response to “Seasonal calibration of the end-cretaceous Chicxulub impact event.” OSF Preprints, fu7rp, ver. 5, peer-reviewed by PCI Paleo. https://doi.org/10.31219/osf.io/fu7rp Price, M. (2022). Paleontologist accused of fraud in paper on dino-killing asteroid. Science, 378(6625), 1155–1157. https://doi.org/10.1126/science.adg2855 Price, M. (2023). Dinosaur extinction researcher guilty of research misconduct. Science, 382(6676), 1225–1225. https://doi.org/10.1126/science.adn4967 | Calibrations without raw data - a response to "Seasonal calibration of the end-cretaceous Chicxulub impact event" | Melanie A.D. During, Dennis F.A.E. Voeten, Per E. Ahlberg | <p>A recent paper by DePalma et al. reported that the season of the End-Cretaceous mass extinction was confined to spring/summer on the basis of stable isotope analyses and supplementary observations. An independent study that was concurrently und... | Fossil calibration, Geochemistry, Methods, Vertebrate paleontology | Christina Belanger | 2023-06-22 10:43:31 | View | ||
27 Jan 2020

A simple generative model of trilobite segmentation and growthMelanie J Hopkins https://doi.org/10.31233/osf.io/zt642Deep insights into trilobite developmentRecommended by Christian Klug based on reviews by Kenneth De Baets and Lukas LaiblTrilobites are arthropods that became extinct at the greatest marine mass extinction over 250 Ma ago. Because of their often bizarre forms, their great diversity and disparity of shapes, they have attracted the interest of researchers and laypersons alike. Due to their calcified exoskeleton, their remains are quite abundant in many marine strata. One particularly interesting aspect, however, is the fossilization of various molting stages. This allows the reconstruction of both juvenile strategies (lecitotrophic versus planktotrophic) and the entire life history of at least some well-documented taxa (e.g., Hughes 2003, 2007; Laibl 2017). For example, life history of trilobites is, based on certain morphological changes, classically subdivided in the three phases protaspis (hatchling, one dorsal shield with few segments with no articulation between), meraspis (juvenile, two and more shields connected by articulations) and holaspis (when the terminal number of thoracic segments is reached). At most molting events, a new skeletal element is added (only in the holaspis, the number of thoracic segments does not change). Nevertheless, many trilobites are known mainly from late meraspid and holaspid stages, because the dorsal shields of the first ontogenetic stages are usually very small and thus often either dissolved or overlooked. An improved understanding of trilobite ontogeny could thus help filling in these gaps in fossil preservation and subsequently, to better understand evolutionary pathways. This is where this paper comes in. In a very clever approach, the New-York-based researcher Melanie Hopkins modeled the growth of these segmented animals (Hopkins 2020). Previous growth models of invertebrates focused on, e.g., mollusks, whose shells grow by accretion. Modelling arthropod ontogeny represented a challenge, which is now overcome by Hopkins' brilliant paper. Her generative growth model is based on empirical data of Aulacopleura koninckii (Barrande, 1846). Hong et al. (2014) and Hughes et al. (2017) documented the ontogeny of this 429 Ma old trilobite species in great detail. In the Silurian of the Barrandian region (Czech Republic), this species is locally very common and all growth stages are well known. I could imagine that the paper of Hughes et al. (2017) planted the seed into Melanie Hopkins’ mind to approach trilobite development in general in a quantitative way with a mathematical approach comparable to the mollusk-research by, e.g., David Raup (1961, 1966) and George McGhee (2015). Hopkins’ growth model requires “a minimum of nine parameters […] to model basic trilobite growth and segmentation, and three additional parameters […] to allow a transition to a new growth gradient for the trunk region during ontogeny” (Hopkins 2020: p. 21). It is now possible to play with parameters such as protaspid size, segment dimensions, segment numbers, etc., in order to estimate changes in body size or morphology. Furthermore, the model could be applied to similarly organized arthropod exoskeletons like many early Cambrian arthropods (e.g., marellomorphs) or even crustaceans (e.g., conchostracans or copepods). Of great interest could also be to assess influences of environmental changes on arthropod ontogeny. Also, her work will help to reconstruct unknown developmental information missing from trilobite species (and possibly other arthropods) and also to explore their morphospace. References Barrande, J. (1846). Notice préliminaire sur le système Silurien et les trilobites de Bohême. Leipzig: Hirschfield.
Hong, P. S., Hughes, N. C., & Sheets, H. D. (2014). Size, shape, and systematics of the Silurian trilobite Aulacopleura koninckii. Journal of Paleontology, 88(6), 1120–1138. doi: 10.1666/13-142 | A simple generative model of trilobite segmentation and growth | Melanie J Hopkins | <p>Generative growth models have been the basis for numerous studies of morphological diversity and evolution. Most work has focused on modeling accretionary growth systems, with much less attention to discrete growth systems. Generative growth mo... |  | Evo-Devo, Evolutionary biology, Invertebrate paleontology, Paleobiology | Christian Klug | 2019-10-06 00:27:25 | View | |
07 Mar 2024
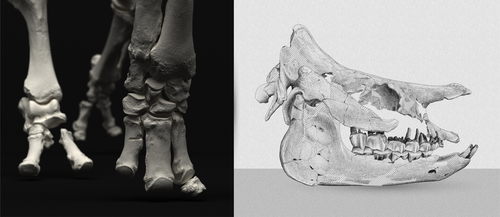
An Early Miocene skeleton of Brachydiceratherium Lavocat, 1951 (Mammalia, Perissodactyla) from the Baikal area, Russia, and a revised phylogeny of Eurasian teleoceratinesAlexander Sizov, Alexey Klementiev, Pierre-Olivier Antoine https://doi.org/10.1101/2022.07.06.498987A Rhino from Lake BaikalRecommended by Faysal Bibi based on reviews by Jérémy Tissier, Panagiotis Kampouridis and Tao Deng based on reviews by Jérémy Tissier, Panagiotis Kampouridis and Tao Deng
As for many groups, such as equids or elephants, the number of living rhinoceros species is just a fraction of their past diversity as revealed by the fossil record. Besides being far more widespread and taxonomically diverse, rhinos also came in a greater variety of shapes and sizes. Some of these – teleoceratines, or so-called ‘hippo-like’ rhinos – had short limbs, barrel-shaped bodies, were often hornless, and might have been semi-aquatic (Prothero et al., 1989; Antoine, 2002). Teleoceratines existed from the Oligocene to the Pliocene, and have been recorded from Eurasia, Africa, and North and Central America. Despite this large temporal and spatial presence, large gaps remain in our knowledge of this group, particularly when it comes to their phylogeny and their relationships to other parts of the rhino tree (Antoine, 2002; Lu et al., 2021). Here, Sizov et al. (2024) describe an almost complete skeleton of a teleoceratine found in 2008 on an island in Lake Baikal in eastern Russia. Dating to the Early Miocene, this wonderfully preserved specimen includes the skull and limb bones, which are described and figured in detail, and which indicate assignment to Brachydiceratherium shanwangense, a species otherwise known only from Shandong in eastern China, some 2000 km to the southeast (Wang, 1965; Lu et al., 2021). The study goes on to present a new phylogenetic analysis of the teleoceratines, the results of which have important implications for the taxonomy of fossil rhinos. Besides confirming the monophyly of Teleoceratina, the phylogeny supports the reassignment of most species previously assigned to Diaceratherium to Brachydiceratherium instead. In a field that is increasingly dominated by analyses of metadata, Sizov et al. (2024) provide a reminder of the importance of fieldwork for the discovery of fossil remains that, sometimes by virtue of a single specimen, can significantly augment our understanding of the evolution and paleobiogeography of extinct species. References Antoine, P.-O. (2002). Phylogénie et évolution des Elasmotheriina (Mammalia, Rhinocerotidae). Mémoires du Muséum National d’Histoire Naturelle, 188, 1–359. Lu, X., Cerdeño, E., Zheng, X., Wang, S., & Deng, T. (2021). The first Asian skeleton of Diaceratherium from the early Miocene Shanwang Basin (Shandong, China), and implications for its migration route. Journal of Asian Earth Sciences: X, 6, 100074. https://doi.org/10.1016/j.jaesx.2021.100074 Prothero, D. R., Guérin, C., and Manning, E. (1989). The History of the Rhinocerotoidea. In D. R. Prothero and R. M. Schoch (Eds.), The Evolution of Perissodactyls (pp. 322–340). Oxford University Press. Sizov, A., Klementiev, A., & Antoine, P.-O. (2024). An Early Miocene skeleton of Brachydiceratherium Lavocat, 1951 (Mammalia, Perissodactyla) from the Baikal area, Russia, and a revised phylogeny of Eurasian teleoceratines. bioRxiv, 498987, ver. 6 peer-reviewed by PCI Paleo. https://doi.org/10.1101/2022.07.06.498987 Wang, B. Y. (1965). A new Miocene aceratheriine rhinoceros of Shanwang, Shandong. Vertebrata Palasiatica, 9, 109–112.
| An Early Miocene skeleton of *Brachydiceratherium* Lavocat, 1951 (Mammalia, Perissodactyla) from the Baikal area, Russia, and a revised phylogeny of Eurasian teleoceratines | Alexander Sizov, Alexey Klementiev, Pierre-Olivier Antoine | <p>Hippo-like rhinocerotids, or teleoceratines, were a conspicuous component of Holarctic Miocene mammalian faunas, but their phylogenetic relationships remain poorly known. Excavations in lower Miocene deposits of the Olkhon Island (Tagay localit... |  | Biostratigraphy, Comparative anatomy, Fieldwork, Paleobiogeography, Paleogeography, Phylogenetics, Systematics, Vertebrate paleontology | Faysal Bibi | 2022-07-07 15:27:12 | View | |
01 Oct 2021

Ammonoid taxonomy with supervised and unsupervised machine learning algorithmsFloe Foxon https://doi.org/10.31233/osf.io/ewkx9Performance of machine-learning approaches in identifying ammonoid species based on conch propertiesRecommended by Kenneth De Baets based on reviews by Jérémie Bardin and 1 anonymous reviewerThere are less and less experts on taxonomy of particular groups particularly among early career paleontologists and (paleo)biologists – this also includes ammonoid cephalopods. Techniques cannot replace this taxonomic expertise (Engel et al. 2021) but machine learning approaches can make taxonomy more efficient, reproducible as well as passing it over more sustainable. Initially ammonoid taxonomy was a black box with small differences sometimes sufficient to erect different species as well as really idiosyncratic groupings of superficially similar specimens (see De Baets et al. 2015 for a review). In the meantime, scientists have embraced more quantitative assessments of conch shape and morphology more generally (see Klug et al. 2015 for a more recent review). The approaches still rely on important but time-intensive collection work and seeing through daisy chains of more or less accessible papers and monographs without really knowing how these approaches perform (other than expert opinion). In addition, younger scientists are usually trained by more experienced scientists, but this practice is becoming more and more difficult which makes it difficult to resolve the taxonomic gap. This relates to the fact that less and less experienced researchers with this kind of expertise get employed as well as graduate students or postdocs choosing different research or job avenues after their initial training effectively leading to a leaky pipeline and taxonomic impediment. Robust taxonomy and stratigraphy is the basis for all other studies we do as paleontologists/paleobiologists so Foxon (2021) represents the first step to use supervised and unsupervised machine-learning approaches and test their efficiency on ammonoid conch properties. This pilot study demonstrates that machine learning approaches can be reasonably accurate (60-70%) in identifying ammonoid species (Foxon, 2021) – at least similar to that in other mollusk taxa (e.g., Klinkenbuß et al. 2020) - and might also be interesting to assist in cases where more traditional methods are not feasible. Novel approaches might even allow to further approve the accuracy as has been demonstrated for other research objects like pollen (Romero et al. 2020). Further applying of machine learning approaches on larger datasets and additional morphological features (e.g., suture line) are now necessary in order to test and improve the robustness of these approaches for ammonoids as well as test their performance more broadly within paleontology.
References De Baets K, Bert D, Hoffmann R, Monnet C, Yacobucci M, and Klug C (2015). Ammonoid intraspecific variability. In: Ammonoid Paleobiology: From anatomy to ecology. Ed. by Klug C, Korn D, De Baets K, Kruta I, and Mapes R. Vol. 43. Topics in Geobiology. Dordrecht: Springer, pp. 359–426. Engel MS, Ceríaco LMP, Daniel GM, Dellapé PM, Löbl I, Marinov M, Reis RE, Young MT, Dubois A, Agarwal I, Lehmann A. P, Alvarado M, Alvarez N, Andreone F, Araujo-Vieira K, Ascher JS, Baêta D, Baldo D, Bandeira SA, Barden P, Barrasso DA, Bendifallah L, Bockmann FA, Böhme W, Borkent A, Brandão CRF, Busack SD, Bybee SM, Channing A, Chatzimanolis S, Christenhusz MJM, Crisci JV, D’elía G, Da Costa LM, Davis SR, De Lucena CAS, Deuve T, Fernandes Elizalde S, Faivovich J, Farooq H, Ferguson AW, Gippoliti S, Gonçalves FMP, Gonzalez VH, Greenbaum E, Hinojosa-Díaz IA, Ineich I, Jiang J, Kahono S, Kury AB, Lucinda PHF, Lynch JD, Malécot V, Marques MP, Marris JWM, Mckellar RC, Mendes LF, Nihei SS, Nishikawa K, Ohler A, Orrico VGD, Ota H, Paiva J, Parrinha D, Pauwels OSG, Pereyra MO, Pestana LB, Pinheiro PDP, Prendini L, Prokop J, Rasmussen C, Rödel MO, Rodrigues MT, Rodríguez SM, Salatnaya H, Sampaio Í, Sánchez-García A, Shebl MA, Santos BS, Solórzano-Kraemer MM, Sousa ACA, Stoev P, Teta P, Trape JF, Dos Santos CVD, Vasudevan K, Vink CJ, Vogel G, Wagner P, Wappler T, Ware JL, Wedmann S, and Zacharie CK (2021). The taxonomic impediment: a shortage of taxonomists, not the lack of technical approaches. Zoological Journal of the Linnean Society 193, 381–387. doi: 10. 1093/zoolinnean/zlab072 Foxon F (2021). Ammonoid taxonomy with supervised and unsupervised machine learning algorithms. PaleorXiv ewkx9, ver. 3, peer-reviewed by PCI Paleo. doi: 10.31233/osf.io/ewkx9 Klinkenbuß D, Metz O, Reichert J, Hauffe T, Neubauer TA, Wesselingh FP, and Wilke T (2020). Performance of 3D morphological methods in the machine learning assisted classification of closely related fossil bivalve species of the genus Dreissena. Malacologia 63, 95. doi: 10.4002/040.063.0109 Klug C, Korn D, Landman NH, Tanabe K, De Baets K, and Naglik C (2015). Ammonoid conchs. In: Ammonoid Paleobiology: From anatomy to ecology. Ed. by Klug C, Korn D, De Baets K, Kruta I, and Mapes RH. Vol. 43. Dordrecht: Springer, pp. 3–24. Romero IC, Kong S, Fowlkes CC, Jaramillo C, Urban MA, Oboh-Ikuenobe F, D’Apolito C, and Punyasena SW (2020). Improving the taxonomy of fossil pollen using convolutional neural networks and superresolution microscopy. Proceedings of the National Academy of Sciences 117, 28496–28505. doi: 10.1073/pnas.2007324117 | Ammonoid taxonomy with supervised and unsupervised machine learning algorithms | Floe Foxon | <p>Ammonoid identification is crucial to biostratigraphy, systematic palaeontology, and evolutionary biology, but may prove difficult when shell features and sutures are poorly preserved. This necessitates novel approaches to ammonoid taxonomy. Th... |  | Invertebrate paleontology, Taxonomy | Kenneth De Baets | Jérémie Bardin | 2021-01-06 11:48:35 | View |
13 Jul 2023
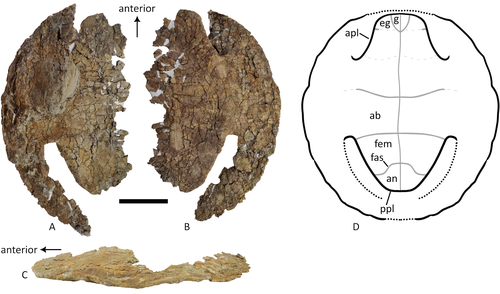
A baenid turtle shell from the Mesaverde Formation (Campanian, Late Cretaceous) of Park County, Wyoming, USAKa Yan Wu, Jared Heuck, Frank J. Varriale, and Andrew A. Farke https://doi.org/10.31233/osf.io/uk3acNew baenid turtle material from the Campanian of WyomingRecommended by Jérémy Anquetin based on reviews by Heather F. Smith and Brent Adrian based on reviews by Heather F. Smith and Brent Adrian
The Baenidae form a diverse extinct clade of exclusively North American paracryptodiran turtles known from the Early Cretaceous to the Eocene (Hay, 1908; Gaffney, 1972; Joyce and Lyson, 2015). Their fossil record was recently extended down to the Berriasian-Valanginian (Joyce et al. 2020), but the group probably originates in the Late Jurassic because it is usually retrieved as the sister group of Pleurosternidae in phylogenetic analyses. However, baenids only became abundant during the Late Cretaceous, when they are restricted in distribution to the western United States, Alberta and Saskatchewan (Joyce and Lyson, 2015). During the Campanian, baenids are abundant in the northern (Alberta, Montana) and southern (Texas, New Mexico, Utah) parts of their range, but in the middle part of this range they are mostly represented by poorly diagnosable shell fragments. In their new contribution, Wu et al. (2023) describe a new articulated baenid specimen from the Campanian Mesaverde Formation of Wyoming. Despite its poor preservation, they are able to confidently assign this partial shell to Neurankylus sp., hence definitively confirming the presence of baenids and Neurankylus in this formation. Incidentally, this new specimen was found in a non-fluvial depositional environment, which would also confirm the interpretation of Neurankylus as a pond turtle (Hutchinson and Archibald, 1986; Sullivan et al., 1988; Wu et al., 2023; see also comments from the second reviewer). The study of Wu et al. (2023) also includes a detailed account of the state of the fossil when it was discovered and the subsequent extraction and preparation procedures followed by the team. This may seem excessive or out of place to some, but I agree with the authors that such information, when available, should be more commonly integrated into scientific articles describing new fossil specimens. Preparation and restoration can have a significant impact on the perceived morphology. This must be taken into account when working with fossil specimens. The chemicals or products used to treat, prepare, or consolidate the specimens are also important information for long-term curation. Therefore, it is important that such information is recorded and made available for researchers, curators, and preparators. References Gaffney, E. S. (1972). The systematics of the North American family Baenidae (Reptilia, Cryptodira). Bulletin of the American Museum of Natural History, 147(5), 241–320. Hay, O. P. (1908). The Fossil Turtles of North America. Carnegie Institution of Washington, Washington, D.C. https://doi.org/10.5962/bhl.title.12500 Hutchison, J. H., and Archibald, J. D. (1986). Diversity of turtles across the Cretaceous/Tertiary boundary in Northeastern Montana. Palaeogeography, Palaeoclimatology, Palaeoecology, 55(1), 1–22. https://doi.org/10.1016/0031-0182(86)90133-1 Joyce, W. G., and Lyson, T. R. (2015). A review of the fossil record of turtles of the clade Baenidae. Bulletin of the Peabody Museum of Natural History, 56(2), 147–183. https://doi.org/10.3374/014.058.0105 Joyce, W. G., Rollot, Y., and Cifelli, R. L. (2020). A new species of baenid turtle from the Early Cretaceous Lakota Formation of South Dakota. Fossil Record, 23(1), 1–13. https://doi.org/10.5194/fr-23-1-2020 Sullivan, R. M., Lucas, S. G., Hunt, A. P., and Fritts, T. H. (1988). Color pattern on the selmacryptodiran turtle Neurankylus from the Early Paleocene (Puercan) of the San Juan Basin, New Mexico. Contributions in Science, 401, 1–9. https://doi.org/10.5962/p.241286 Wu, K. Y., Heuck, J., Varriale, F. J., and Farke, A. (2023). A baenid turtle shell from the Mesaverde Formation (Campanian, Late Cretaceous) of Park County, Wyoming, USA. PaleorXiv, uk3ac, ver. 5, peer-reviewed and recommended by Peer Community In Paleontology. https://doi.org/10.31233/osf.io/uk3ac | A baenid turtle shell from the Mesaverde Formation (Campanian, Late Cretaceous) of Park County, Wyoming, USA | Ka Yan Wu, Jared Heuck, Frank J. Varriale, and Andrew A. Farke | <p>The Mesaverde Formation of the Wind River and Bighorn basins of Wyoming preserves a rich yet relatively unstudied terrestrial and marine faunal assemblage dating to the Campanian. To date, turtles within the formation have been represented prim... |  | Paleobiodiversity, Paleobiogeography, Vertebrate paleontology | Jérémy Anquetin | 2023-01-16 16:26:43 | View |
MANAGING BOARD
Jérémy Anquetin
Faysal Bibi
Guillaume Billet
Andrew A. Farke
Franck Guy
Leslea J. Hlusko
Melanie Hopkins
Cynthia V. Looy
Jesús Marugán-Lobón
Ilaria Mazzini
P. David Polly
Caroline A.E. Strömberg










