Latest recommendations

| Id | Title * | Authors * ▲ | Abstract * | Picture * | Thematic fields * | Recommender | Reviewers | Submission date | |
|---|---|---|---|---|---|---|---|---|---|
04 Jun 2024

New generic name for a small Triassic ray-finned fish from Perledo (Italy)Adriana López-Arbarello, Rainer Brocke https://doi.org/10.31233/osf.io/bxmg5A new study on the halecomorph fishes from the Triassic of Perledo (Italy) highlights important issues in PalaeoichthyologyRecommended by Hugo Martín Abad based on reviews by Guang-Hui Xu and 1 anonymous reviewerMesozoic fishes are extremely diverse. In fact, fishes are the most diverse group of vertebrates during the Mesozoic─just as during any other era. Yet, their study is severely underrepresented in comparison to other fossil groups. There are just too few palaeoichthyologists to deal with such a vast diversity of fishes. Nonetheless, thanks to the huge efforts they have made over the last few decades, we have come a long way in our understanding of Mesozoic ichthyofaunas. One of such devoted palaeoichthyologists is Dr. Adriana López-Arbarello, whose contributions have been crucial in elucidating the phylogenetic interrelationships and taxonomic diversity of Mesozoic actinopterygian fishes (e.g., López-Arbarello, 2012; López-Arbarello & Sferco, 2018; López-Arbarello & Ebert, 2023). In her most recent manuscript, Dr. López-Arbarello has joined forces with Dr. Rainer Brocke to tackle the taxonomy and systematics of the halecomorph fishes from one of the most relevant Triassic sites, the upper Ladinian Perledo locality from Italy (López-Arbarello & Brocke, 2024). Fossil fishes were reported for the first time from Perledo in the first half of the 19th century (Balsamo-Crivelli, 1839), and up to 30 different species were described from the locality in the subsequent decades. Unfortunately, this is one of the multiple examples of fossil collections that suffered the effects of World War II, and most of the type material was lost. As a consequence, many of those 30 species that have been described over the years are in need of a revision. Based on the study of additional material that was transferred to Germany and is housed at the Senckenberg Research Institute and Natural History Museum, López-Arbarello & Brocke (2024) confirm the presence of four different species of halecomorph fishes in Perledo, which were previously put under synonymy (Lombardo, 2001). They provide new detailed information on the anatomy of two of those species, together with their respective diagnoses. But more importantly, they carry out a thorough exercise of taxonomy, rigorously applying the International Code of Zoological Nomenclature to disentangle the intricacies in the taxonomic story of the species placed in the genus Allolepidotus. As a result, they propose the presence of the species A. ruppelii, which they propose to be the type species for that genus (instead of A. bellottii, which they transfer to the genus Eoeugnathus). They also propose a new genus for the other species originally included in Allolepidotus, A. nothosomoides. Finally, they provide a set of measurements and ratios for Pholidophorus oblongus and Pholidophorus curionii, the other two species previously put in synonymy with A. bellottii, to demonstrate their validity as different species. However, due to the loss of the type material, the authors propose that these two species remain as nomina dubia. In summary, apart from providing new detailed anatomical descriptions of two species and solving some long-standing issues with the taxonomy of the halecomorphs from the relevant Triassic Perledo locality, the paper by López-Arbarello & Rainer (2024) highlights three important topics for the study of the fossil record: 1) we should never forget that world-scale problems, such as World Wars, also affect our capacity to understand the natural world in which we live, and the whole society should be aware if this; 2) the importance of exhaustively following the International Code of Zoological Nomenclature when describing new species; and 3) we are in need of new palaeoichthyologists to, in Dr. López-Arbarello’s own words, “unveil the mysteries of those marvellous Mesozoic ichthyofaunas.” References Balsamo-Crivelli, G. (1839). Descrizione di un nuovo rettile fossile, della famiglia dei Paleosauri, e di due pesci fossili, trovati nel calcare nero, sopra Varenna sul lago di Como, dal nobile sig. Ludovico Trotti, con alcune riflessioni geologiche. Il politecnico repertorio mensile di studj applicati alla prosperita e coltura sociale, 1, 421–431. Lombardo, C. (2001). Actinopterygians from the Middle Triassic of northern Italy and Canton Ticino (Switzerland): Anatomical descriptions and nomenclatural problems. Rivista Italiana di Paleontologia e Stratigrafia, 107, 345–369. https://doi.org/10.13130/2039-4942/5439 López-Arbarello, A. (2012). Phylogenetic interrelationships of ginglymodian fishes (Actinopterygii: Neopterygii). PLOS ONE, 7(7), e39370. https://doi.org/10.1371/journal.pone.0039370 López-Arbarello, A., and Brocke, R. (2024). New generic name for a small Triassic ray-finned fish from Perledo (Italy). PaleorXiv, bxmg5, ver. 4, peer-reviewed by PCI Paleo. https://doi.org/10.31233/osf.io/bxmg5 López-Arbarello, A., and Ebert, M. (2023). Taxonomic status of the caturid genera (Halecomorphi, Caturidae) and their Late Jurassic species. Royal Society Open Science, 10(1), 221318. https://doi.org/10.1098/rsos.221318 López-Arbarello, A., and Sferco, E. (2018). Neopterygian phylogeny: The merger assay. Royal Society Open Science, 5(3), 172337. https://doi.org/10.1098/rsos.172337 | New generic name for a small Triassic ray-finned fish from Perledo (Italy) | Adriana López-Arbarello, Rainer Brocke | <p>Our new study of the species originally included in the genus <em>Allolepidotus</em> led to the taxonomic revision of the halecomorph species from the Triassic of Perledo, Italy. The morphological variation revealed by the analysis of the type ... |  | Fossil record, Systematics, Taxonomy, Vertebrate paleontology | Hugo Martín Abad | 2024-03-21 11:53:53 | View | |
07 Mar 2024
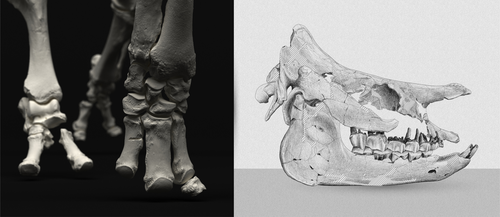
An Early Miocene skeleton of Brachydiceratherium Lavocat, 1951 (Mammalia, Perissodactyla) from the Baikal area, Russia, and a revised phylogeny of Eurasian teleoceratinesAlexander Sizov, Alexey Klementiev, Pierre-Olivier Antoine https://doi.org/10.1101/2022.07.06.498987A Rhino from Lake BaikalRecommended by Faysal Bibi based on reviews by Jérémy Tissier, Panagiotis Kampouridis and Tao Deng based on reviews by Jérémy Tissier, Panagiotis Kampouridis and Tao Deng
As for many groups, such as equids or elephants, the number of living rhinoceros species is just a fraction of their past diversity as revealed by the fossil record. Besides being far more widespread and taxonomically diverse, rhinos also came in a greater variety of shapes and sizes. Some of these – teleoceratines, or so-called ‘hippo-like’ rhinos – had short limbs, barrel-shaped bodies, were often hornless, and might have been semi-aquatic (Prothero et al., 1989; Antoine, 2002). Teleoceratines existed from the Oligocene to the Pliocene, and have been recorded from Eurasia, Africa, and North and Central America. Despite this large temporal and spatial presence, large gaps remain in our knowledge of this group, particularly when it comes to their phylogeny and their relationships to other parts of the rhino tree (Antoine, 2002; Lu et al., 2021). Here, Sizov et al. (2024) describe an almost complete skeleton of a teleoceratine found in 2008 on an island in Lake Baikal in eastern Russia. Dating to the Early Miocene, this wonderfully preserved specimen includes the skull and limb bones, which are described and figured in detail, and which indicate assignment to Brachydiceratherium shanwangense, a species otherwise known only from Shandong in eastern China, some 2000 km to the southeast (Wang, 1965; Lu et al., 2021). The study goes on to present a new phylogenetic analysis of the teleoceratines, the results of which have important implications for the taxonomy of fossil rhinos. Besides confirming the monophyly of Teleoceratina, the phylogeny supports the reassignment of most species previously assigned to Diaceratherium to Brachydiceratherium instead. In a field that is increasingly dominated by analyses of metadata, Sizov et al. (2024) provide a reminder of the importance of fieldwork for the discovery of fossil remains that, sometimes by virtue of a single specimen, can significantly augment our understanding of the evolution and paleobiogeography of extinct species. References Antoine, P.-O. (2002). Phylogénie et évolution des Elasmotheriina (Mammalia, Rhinocerotidae). Mémoires du Muséum National d’Histoire Naturelle, 188, 1–359. Lu, X., Cerdeño, E., Zheng, X., Wang, S., & Deng, T. (2021). The first Asian skeleton of Diaceratherium from the early Miocene Shanwang Basin (Shandong, China), and implications for its migration route. Journal of Asian Earth Sciences: X, 6, 100074. https://doi.org/10.1016/j.jaesx.2021.100074 Prothero, D. R., Guérin, C., and Manning, E. (1989). The History of the Rhinocerotoidea. In D. R. Prothero and R. M. Schoch (Eds.), The Evolution of Perissodactyls (pp. 322–340). Oxford University Press. Sizov, A., Klementiev, A., & Antoine, P.-O. (2024). An Early Miocene skeleton of Brachydiceratherium Lavocat, 1951 (Mammalia, Perissodactyla) from the Baikal area, Russia, and a revised phylogeny of Eurasian teleoceratines. bioRxiv, 498987, ver. 6 peer-reviewed by PCI Paleo. https://doi.org/10.1101/2022.07.06.498987 Wang, B. Y. (1965). A new Miocene aceratheriine rhinoceros of Shanwang, Shandong. Vertebrata Palasiatica, 9, 109–112.
| An Early Miocene skeleton of *Brachydiceratherium* Lavocat, 1951 (Mammalia, Perissodactyla) from the Baikal area, Russia, and a revised phylogeny of Eurasian teleoceratines | Alexander Sizov, Alexey Klementiev, Pierre-Olivier Antoine | <p>Hippo-like rhinocerotids, or teleoceratines, were a conspicuous component of Holarctic Miocene mammalian faunas, but their phylogenetic relationships remain poorly known. Excavations in lower Miocene deposits of the Olkhon Island (Tagay localit... |  | Biostratigraphy, Comparative anatomy, Fieldwork, Paleobiogeography, Paleogeography, Phylogenetics, Systematics, Vertebrate paleontology | Faysal Bibi | 2022-07-07 15:27:12 | View | |
20 Oct 2020
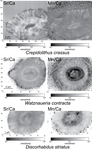
Evidence of high Sr/Ca in a Middle Jurassic murolith coccolith speciesBaptiste Suchéras-Marx, Fabienne Giraud, Alexandre Simionovici, Rémi Tucoulou, Isabelle Daniel https://doi.org/10.31233/osf.io/dcfuqNew results and challenges in Sr/Ca studies on Jurassic coccolithophoridsRecommended by Antonino Briguglio based on reviews by Kenneth De Baets and 1 anonymous reviewerThis interesting publication by Suchéras-Marx et al. (2020) highlights peculiar aspects of geochemistry in nannofossils, specifically coccolithophorids. One of the main application of geochemistry on fossil shells is to get hints on the physiology of such extinct taxa. Here, the authors try to get information on the calcification mechanism and processes in Jurassic coccoliths. Coccoliths build a test made of calcium carbonate and one of the most common geochemical proxies used for this fossil group is the Sr/Ca ratio. This isotopic ratio has good chances to be successfully used as a robust proxy for paleoenvironmental reconstruction, but, concerning Jurassic coccoliths things seem to be not straightforward. The authors managed to compare the isotopic value of Sr/Ca measured on Jurassic coccoliths from different taxonomic groups: the murolith Crepidolithus crassus and the placoliths Watznaueria contracta and Discorhabdus striatus. The results they got clearly show that the Sr/Ca ratio cannot be used as a universal proxy because these species exhibit very different values despite coming from the same stratigraphic level and having undergone minimal diagenetic modification. Data seem to point to a Sr/Ca ratio up to 10 times higher in the murolith species than in the placolith taxa (Suchéras-Marx et al., 2020). One of the explanation given here takes advantage of modern coccolith data and hints to specific polysaccharides that would control the growth of the long R unit in the murolith species. As always, there is plenty of space for additional research, possibly on modern taxa, to sort out the scientific questions that arise from this work. References Suchéras-Marx, B., Giraud, F., Simionovici, A., Tucoulou, R., & Daniel, I. (2020). Evidence of high Sr/Ca in a Middle Jurassic murolith coccolith species. PaleorXiv, dcfuq, version 7, peer-reviewed by PCI Paleo. doi: 10.31233/osf.io/dcfuq | Evidence of high Sr/Ca in a Middle Jurassic murolith coccolith species | Baptiste Suchéras-Marx, Fabienne Giraud, Alexandre Simionovici, Rémi Tucoulou, Isabelle Daniel | <p>Paleoceanographical reconstructions are often based on microfossil geochemical analyses. Coccoliths are the most ancient, abundant and continuous record of pelagic photic zone calcite producer organisms. Hence, they could be valuable substrates... |  | Microfossils, Micropaleontology, Nanofossils | Antonino Briguglio | 2020-05-18 16:11:35 | View | |
26 Oct 2023
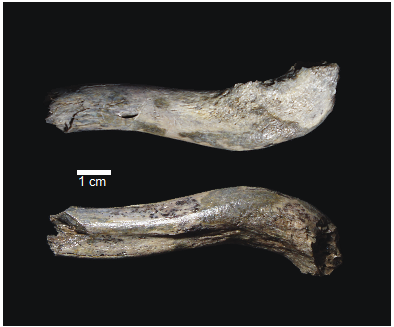
OH 89: A newly described ~1.8-million-year-old hominid clavicle from Olduvai GorgeCatherine E. Taylor, Fidelis Masao, Jackson K. Njau, Agustino Venance Songita, Leslea J. Hlusko https://doi.org/10.1101/2023.02.02.526656A new method for measuring clavicular curvatureRecommended by Nuria Garcia based on reviews by 2 anonymous reviewersThe evolution of the hominid clavicle has not been studied in depth by paleoanthropologists given its high morphological variability and the scarcity of complete diagnosable specimens. A nearly complete Nacholapithecus clavicle from Kenya (Senut et al. 2004) together with a fragment from Ardipithecus from the Afar region of Ethiopia (Lovejoy et al. 2009) complete our knowledge of the Miocene record. The Australopithecus collection of clavicles from Eastern and South African Plio-Pleistocene sites is slightly more abundant but mostly represented by fragmentary specimens. The number of fossil clavicles increases for the genus Homo from more recent sites and thus our potential knowledge about the shoulder evolution. In their new contribution, Taylor et al. (2023) present a detailed analysis of OH 89, a ~1.8-million-year-old partial hominin clavicle recovered from Olduvai Gorge (Tanzania). The work goes over previous studies which included clavicles found in the hominid fossil record. The text is accompanied by useful tables of data and a series of excellent photographs. It is a great opportunity to learn its role in the evolution of the hominid shoulder gird as clavicles are relatively poorly preserved in the fossil record compared to other long bones. The study compares the specimen OH 89 with five other hominid clavicles and a sample of 25 modern clavicles, 30 Gorilla, 31 Pan and 7 Papio. The authors propose a new methodology for measuring clavicular curvature using measurements of sternal and acromial curvature, from which an overall curvature measurement is calculated. The study of OH 89 provides good evidence about the hominid who lived 1.8 million years ago in the Olduvai Gorge region. This time period is especially relevant because it can help to understand the morphological changes that occurred between Australopithecus and the appearance of Homo. The authors conclude that OH 89 is the largest of the hominid clavicles included in the analysis. Although they are not able to assign this partial element to species level, this clavicle from Olduvai is at the larger end of the variation observed in Homo sapiens and show similarities to modern humans, especially when analysing the estimated sinusoidal curvature. References Lovejoy, C. O., Suwa, G., Simpson, S. W., Matternes, J. H., and White, T. D. (2009). The Great Divides: Ardipithecus ramidus peveals the postcrania of our last common ancestors with African apes. Science, 326(5949), 73–106. https://doi.org/10.1126/science.1175833 Senut, B., Nakatsukasa, M., Kunimatsu, Y., Nakano, Y., Takano, T., Tsujikawa, H., Shimizu, D., Kagaya, M., and Ishida, H. (2004). Preliminary analysis of Nacholapithecus scapula and clavicle from Nachola, Kenya. Primates, 45(2), 97–104. https://doi.org/10.1007/s10329-003-0073-5 Taylor, C., Masao, F., Njau, J. K., Songita, A. V., and Hlusko, L. J. (2023). OH 89: A newly described ∼1.8-million-year-old hominid clavicle from Olduvai Gorge. bioRxiv, 526656, ver. 6 peer-reviewed by PCI Paleo. https://doi.org/10.1101/2023.02.02.526656 | OH 89: A newly described ~1.8-million-year-old hominid clavicle from Olduvai Gorge | Catherine E. Taylor, Fidelis Masao, Jackson K. Njau, Agustino Venance Songita, Leslea J. Hlusko | <p>Objectives: Here, we describe the morphology and geologic context of OH 89, a ~1.8-million-year-old partial hominid clavicle from Olduvai Gorge, Tanzania. We compare the morphology and clavicular curvature of OH 89 to modern humans, extant apes... |  | Comparative anatomy, Evolutionary biology, Fossil record, Methods, Morphological evolution, Paleoanthropology, Vertebrate paleontology | Nuria Garcia | 2023-02-08 19:45:01 | View | |
19 Aug 2021

Through a glass darkly, but with more understanding of arthropod originRecommended by Tae-Yoon Park based on reviews by Gerhard Scholtz and Jean VannierArthropods constitute 85% of all described animal species on Earth (Brusca et al., 2016), being the most successful animal phylum at present. This phylum did not even have a shabby beginning. The fossil record shows that they were dominating the sea even in the early Cambrian (Caron and Jackson, 2008; Zhao et al., 2014; Fu et al., 2019). This planet is and has been indeed dominated by arthropods. Within the context of the Big Bang of animal evolution known as the Cambrian Explosion, delving into the origin of arthropods has been one of the all-time fascinating research themes in paleontology, and discussions on the ‘origin’ and ‘early evolution’ of arthropods from the paleontological perspective have not been infrequent (e.g., Budd and Telford, 2009; Edgecombe and Legg, 2014; Daley et al., 2018; Edgecombe, 2020). In this context, Aria (2021) provides an interesting integration of his view on arthropod origin and early evolution. Based on well-preserved Burgess Shale materials, together with other impressive researches mainly with Jean-Bernard Caron (e.g., Aria and Caron, 2015; Caron and Aria, 2017), Cédric Aria made his name known with papers searching for the stem-groups of the two major euarthropod lineages, the Mandibulata and the Chelicerata (Aria and Caron, 2017, 2019), for which (and for his subsequent researches) assembling a large dataset for arthropod phylogeny was inevitable. Subsequently, there have been several major discoveries which could improve our understanding on early arthropod evolution (e.g., Lev and Chipman, 2020; Liu et al., 2020; Zeng et al., 2020). For Cédric Aria, therefore, this timely presentation of his own perspective on the origin of early evolution of arthropods may have been preordain. This review stands out because it discusses almost all aspects of arthropod origin and early evolution that can be possibly covered by paleontology (many, if not all, of which are still controversial). Some of his views may be considered a brave attempt. For instance, based on the widespread occurrences of suspension feeders, Aria (2021) proposes the early Cambrian “planktonic revolution,” which has been associated rather with the Great Ordovician Biodiversification Event (Servais et al., 2016). Given the presence of lophotrochozoans and echinoderms in which the presence of planktonic larvae was likely to have been one of the most inclusive features, the “early Cambrian planktonic revolution” might be plausible, but I wonder if including arthropods into the “earlier revolution” can be readily acceptable. Arthropods arose from direct-developing ancestors, and crustaceans (the main group with planktonic larvae) are pretty much derived in the arthropod phylogenetic tree. Trilobites, inarguably the best-studied Cambrian arthropods, for example, began with direct-developing benthic protaspides in the early Cambrian and Miaolingian. The first hint of planktonic protaspides appeared in the Furongian, and it was not until the Tremadocian when such indirect developing protaspides began to be widespread (Park and Kihm, 2015), which complies well with the onset of the ‘Ordovician Planktonic Revolution’ in the Furongian, as suggested by Servais et al. (2016). But in general, this review presents well-organized views worth hearing, and since many of the points are subject to debate, this review could be a friendly manual for those who have similar views, while it could form a fresh ground to attack for those who have disparate views. One of the endless debates in arthropod research comes from the arthropod head problem (Budd, 2002), which centers on the homology of frontal-most appendages in radiodonts, megacheirans, chelicerates, and mandibulates, as well as on the hypostome-labrum complex. Based on recent interesting discoveries of early arthropods from the Chengjiang biota (Aria, 2020; Zeng et al., 2020), Aria (2021) pertinently coined a term ‘cheirae’ for the frontalmost prehensile appendages of radiodonts and megacheirans, implying homology of them. I assume that not all researchers would agree with this though, as well as with his model of hypostome/labrum complex evolution. Notorious disaccords have also occurred around the phylogenetic positions of early arthropods, such as isoxyids, megacheirans, fuxianhuiids, and artiopodans; different research groups have invariably come up with different topologies. Cédric Aria has presented his own topologies (Aria, 2019, 2020), and figure 2 of Aria (2021) summarizes his perspective very well in combination with major character evolutions. What makes this review especially interesting is a courageous discussion about the origin of biramous appendage, a subject that has been only briefly discussed or overlooked in recent literature. In the current mainstream of this debate lies the concept of ‘gilled lobopodians’ (Budd, 1998), which was complicated by the weird two separate rows of lateral flaps of Aegirocassis (Van Roy et al., 2015). Aria (2021) adds an interesting alternative scenario to this: biramicity originated from the split of main limb axis, as seen in the isoxyid Surusicaris (Aria and Caron, 2015). The figure 3d of his review, therefore, is worth giving thoughts for any arthropodologists who are interested in the origin of arthropod legs. It is true that our understanding of the origin and early evolution of arthropods is still in a mist. Nevertheless, we have recently seen advancements, such as those aided by new types of analysis (Liu et al., 2020), the discoveries of new early arthropods with unexpected morphologies (Aria et al., 2020; Zeng et al., 2020), and the groundbreaking Evo-Devo research (Lev and Chipman, 2020). We will keep jousting on various aspects of the origin and early evolution of arthropods, but for some aspects we are seemingly heading for the final, as implicitly alluded in Aria (2021).
References Aria C (2019). Reviewing the bases for a nomenclatural uniformization of the highest taxonomic levels in arthropods. Geological Magazine 156, 1463–1468. Aria C (2020). Macroevolutionary patterns of body plan canalization in euarthropods. Paleobiology 46, 569–593. doi: 10.1017/pab.2020.36 Aria C (2021). The origin and early evolution of arthropods. PaleorXiv, 4zmey, ver. 4, peer-reviewed by PCI Paleo. doi: 10.31233/osf.io/4zmey Aria C and Caron JB (2015). Cephalic and limb anatomy of a new isoxyid from the Burgess Shale and the role of "stem bivalved arthropods" in the disparity of the frontalmost appendage. PLOS ONE 10, e0124979. doi: 10.1371/journal.pone.0124979 Aria C and Caron JB (2017). Burgess Shale fossils illustrate the origin of the mandibulate body plan. Nature 545, 89–92. Aria C and Caron JB (2019). A middle Cambrian arthropod with chelicerae and proto-book gills. Nature 573, 586–589. doi: 10.1038/s41586-019-1525-4 Aria C, Zhao F, Zeng H, Guo J, and Zhu M (2020). Fossils from South China redefine the ancestral euarthropod body plan. BMC Evolutionary Biology 20, 4. Brusca RC, Moore W, and Shuster SM (2016). Invertebrates. Third edition. Sunderland, Massachusetts U.S.A: Sinauer Associates. isbn: 978-1-60535-375-3 Budd GE (1998). Stem-group arthropods from the Lower Cambrian Sirius Passet fauna of North Greenland. In: Arthropod Relationships. Ed. by Fortey RA and Thomas RH. London, UK: Chapman & Hall, pp. 125–138. Budd GE (2002). A palaeontological solution to the arthropod head problem. Nature 417, 271–275. doi: 10.1038/417271a Budd GE and Telford MJ (2009). The origin and evolution of arthropods. Nature 457, 812–817. doi: 10.1038/Nature07890 Caron JB and Aria C (2017). Cambrian suspension-feeding lobopodians and the early radiation of panarthropods. BMC Evolutionary Biology 17, 29. doi: 10.1186/s12862-016-0858-y Caron JB and Jackson DA (2008). Paleoecology of the Greater Phyllopod Bed community, Burgess Shale. Palaeogeography, Palaeoclimatology, Palaeoecology 258, 222–256. doi: 10.1016/j.palaeo.2007.05.023 Daley AC, Antcliffe JB, Drage HB, and Pates S (2018). Early fossil record of Euarthropoda and the Cambrian Explosion. Proceedings of the National Academy of Sciences of the United States of America 115, 5323–5331. doi: 10.1073/pnas.1719962115 Edgecombe GD (2020). Arthropod origins: Integrating paleontological and molecular evidence. Annual Review of Ecology, Evolution, and Systematics 51, 1–25. doi: 10.1146/annurev-ecolsys-011720-124437 Edgecombe GD and Legg DA (2014). Origins and early evolution of arthropods. Palaeontology 57, 457–468. Fu D, Tong G, Dai T, Liu W, Yang Y, Zhang Y, Cui L, Li L, Yun H, Wu Y, Sun A, Liu C, Pei W, Gaines RR, and Zhang X (2019). The Qingjiang biota—A Burgess Shale–type fossil Lagerstätte from the early Cambrian of South China. Science 363, 1338–1342. doi: 10.1126/science.aau8800 Lev O and Chipman AD (2020). Development of the pre-gnathal segments of the insect head indicates they are not serial homologues of trunk segments. bioRxiv, 2020.09.16.299289. doi: 10.1101/2020.09.16.299289 Liu Y, Ortega-Hernández J, Zhai D, and Hou X (2020). A reduced labrum in a Cambrian great-appendage euarthropod. Current Biology 30, 3057–3061.e2. doi: 10.1016/j.cub.2020.05.085 Park TYS and Kihm JH (2015). Post-embryonic development of the Early Ordovician (ca. 480 Ma) trilobite Apatokephalus latilimbatus Peng, 1990 and the evolution of metamorphosis. Evolution & Development 17, 289–301. doi: 10.1111/ede.12138 Servais T, Perrier V, Danelian T, Klug C, Martin R, Munnecke A, Nowak H, Nützel A, Vandenbroucke TR, Williams M, and Rasmussen CM (2016). The onset of the ‘Ordovician Plankton Revolution’ in the late Cambrian. Palaeogeography, Palaeoclimatology, Palaeoecology 458, 12–28. doi: 10.1016/j.palaeo.2015.11.003 Van Roy P, Daley AC, and Briggs DEG (2015). Anomalocaridid trunk limb homology revealed by a giant filter-feeder with paired flaps. Nature 522, 77–80. doi: 10.1038/nature14256 Zeng H, Zhao F, Niu K, Zhu M, and Huang D (2020). An early Cambrian euarthropod with radiodont-like raptorial appendages. Nature 588, 101–105. doi: 10.1038/s41586-020-2883-7 Zhao F, Caron JB, Bottjer DJ, Hu S, Yin Z, and Zhu M (2014). Diversity and species abundance patterns of the Early Cambrian (Series 2, Stage 3) Chengjiang Biota from China. Paleobiology 40, 50–69. doi: 10.1666/12056 | The origin and early evolution of arthropods | Cédric Aria | <p style="text-align: justify;">The rise of arthropods is a decisive event in the history of life. Likely the first animals to have established themselves on land and in the air, arthropods have pervaded nearly all ecosystems and have become pilla... |  | Comparative anatomy, Evo-Devo, Evolutionary biology, Fossil record, Invertebrate paleontology, Macroevolution, Paleobiodiversity, Paleobiology, Paleoecology, Phylogenetics, Systematics, Taphonomy, Taxonomy | Tae-Yoon Park | 2021-04-26 13:51:21 | View | |
22 Sep 2018
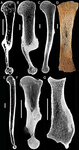
Palaeobiological inferences based on long bone epiphyseal and diaphyseal structure - the forelimb of xenarthrans (Mammalia)Eli Amson & John A. Nyakatura https://doi.org/10.1101/318121Inferences on the lifestyle of fossil xenarthrans based on limb long bone inner structureRecommended by Alexandra Houssaye based on reviews by Andrew Pitsillides and 1 anonymous reviewerBone inner structure bears a strong functional signal and can be used in paleontology to make inferences about the ecology of fossil forms. The increasing use of microtomography enables to analyze both cortical and trabecular features in three dimensions, and thus in long bones to investigate the diaphyseal and epiphyseal structures. Moreover, this can now be done through quantitative, and not only qualitative analyses. Studies focusing on the diaphyseal inner structure (cortical bone and sometimes also spongious bone) of long bones are rather numerous, but essentially based on 2D sections. It is only recently that analyses of the whole diaphyseal structure have been investigated. Studies on the trabecular architecture are much rarer. Amson & Nyakatura (2018) propose a comparative quantitative analysis combining parameters of the epiphyseal trabecular architecture and of the diaphyseal structure, using phylogenetically informed discriminant analyses, and with the aim of inferring the lifestyle of extinct taxa. The group of interest is xenarthrans, one of the four major extant clades of placental mammals. Xenarthrans exhibit different lifestyles, from fully terrestrial to arboreal, and show various degrees of fossoriality. The authors analyzed forelimb long bones of some fossil sloths and made comparisons with several species of extant xenarthrans. The aim was notably to discuss the degree of arboreality and fossoriality of these fossil forms. This study is among the first ones to conjointly analyze both diaphyseal and trabecular parameters to characterize lifestyles, and the first one outside of primates. No fossil form could undoubtedly be assigned to one lifestyle exhibited by extant xenarthrans, though some previous ecological hypotheses could be corroborated. This study also raised some technical challenges, linked to the sample and to the parameters studied, and thus constitutes a great step, from which to go further. References Amson, E., & Nyakatura, J. A. (2018). Palaeobiological inferences based on long bone epiphyseal and diaphyseal structure - the forelimb of xenarthrans (Mammalia). bioRxiv, 318121, ver. 5 peer-reviewed and recommended by PCI Paleo. doi: 10.1101/318121 | Palaeobiological inferences based on long bone epiphyseal and diaphyseal structure - the forelimb of xenarthrans (Mammalia) | Eli Amson & John A. Nyakatura | <p>Trabecular architecture (i.e., the main orientation of the bone trabeculae, their number, mean thickness, spacing, etc.) has been shown experimentally to adapt with great accuracy and sensitivity to the loadings applied to the bone during life.... |  | Biomechanics & Functional morphology, Comparative anatomy, Evolutionary biology, Histology, Methods, Morphological evolution, Paleobiology, Vertebrate paleontology | Alexandra Houssaye | 2018-05-14 08:35:20 | View | |
30 Oct 2019

The Morrison Formation Sauropod Consensus: A freely accessible online spreadsheet of collected sauropod specimens, their housing institutions, contents, references, localities, and other potentially useful informationEmanuel Tschopp, John A. Whitlock, D. Cary Woodruff, John R. Foster, Roberto Lei, Simone Giovanardi https://doi.org/10.31233/osf.io/ydvraSauropods under one (very high) roofRecommended by Jordan Mallon based on reviews by Kenneth Carpenter and Femke HolwerdaFossils get around. Any one fossil locality might be sampled by several collectors from as many institutions around the world. Alternatively, a single collector might heavily sample a site, and sell or trade parts of their collection to other institutions, scattering the fossils far and wide. These practices have the advantage of making fossils from any one locality available to researchers across the globe. However, they also have the disadvantage that, in order to systematically survey any one species, a researcher must follow innumerable trails of breadcrumb to get to where the relevant materials are held. This is true of many famous fossil localities, such as the Eocene Green River Formation in the USA, the Cretaceous Kem Kem beds of Morocco, or the Devonian Miguasha cliffs of Canada. It is especially true of the Upper Jurassic deposits of the Morrison Formation in the western USA, which have yielded an impressive assemblage of megaherbivorous sauropod dinosaurs over the last 150 years. Today, these bones are to be found in museums not just in the USA, but also in Canada, Argentina, Japan, Australia, Malaysia, South Africa, and throughout Europe. Trawling museum databases in search of sauropod material from the Morrison Formation can therefore be a daunting task, never mind traveling the globe to actually study them. A new paper by Tschopp et al. (2019) seeks to ease the burden on sauropod researchers by introducing a database of Morrison Formation sauropods, consisting of over 3000 specimens housed in nearly 40 institutions around the world. The authors are themselves sauropod workers and, having suffered first-hand the plight of studying material from the Morrison Formation, came up with a solution to the problem of keeping track of it all. The database is founded largely on material personally seen by the authors, supplemented by information from the literature and museum catalogs. The database further provides information on bone representation, ontogeny, locality details, and fine-scale stratigraphy, among other fields. Like any database, it is a living document that will continue to grow as new finds are made. Tschopp et al. (2019) have wisely chosen to allow others to contribute to the listing, but changes must first be vetted for accuracy. This product represents 10 years of work, and I have little doubt that it will be well-received by those of us who work on dinosaurs. Speaking personally, my PhD research on megaherbivorous dinosaurs from the Dinosaur Park Formation of Canada led me to institutions in Canada, the USA, and the UK, and further stops to Spain and Argentina would have been beneficial, if affordable. Planning for this work would have been greatly assisted by a database like the one provided us by Tschopp et al. (2019). Many a future graduate student will undoubtedly owe them a debt of gratitude. References Tschopp, E., Whitlock, J. A., Woodruff, D. C., Foster, J. R., Lei, R., & Giovanardi, S. (2019). The Morrison Formation Sauropod Consensus: A freely accessible online spreadsheet of collected sauropod specimens, their housing institutions, contents, references, localities, and other potentially useful information. PaleorXiv, version 3, peer-reviewed by PCI Paleo. doi: 10.31233/osf.io/ydvra | The Morrison Formation Sauropod Consensus: A freely accessible online spreadsheet of collected sauropod specimens, their housing institutions, contents, references, localities, and other potentially useful information | Emanuel Tschopp, John A. Whitlock, D. Cary Woodruff, John R. Foster, Roberto Lei, Simone Giovanardi | <p>The Morrison Formation has been explored for dinosaurs for more than 150 years, in particular for large sauropod skeletons to be mounted in museum exhibits around the world. Several long-term campaigns to the Jurassic West of the United States ... |  | Fossil record, Methods, Paleobiodiversity, Taxonomy, Vertebrate paleontology | Jordan Mallon | 2019-07-19 16:13:45 | View | |
23 Apr 2021

The record of Deinotheriidae from the Miocene of the Swiss Jura Mountains (Jura Canton, Switzerland)Fanny Gagliardi, Olivier Maridet, Damien Becker https://doi.org/10.1101/2020.08.10.244061The fossil record of deinotheres in the Jura Mountains and the specific diversity of European deinotheriidsRecommended by Lionel Hautier based on reviews by Martin Pickford and 1 anonymous reviewerProboscideans belong to the Afrotheria, a superorder of mammals with an African origin, which was recently recognized based on molecular data (see review in Asher et al., 2009). The fossil record of Proboscidea is well documented and shows that an important part of their evolutionary history took place in Africa, with their representatives inhabiting the continent for at least 60 million years (Gheerbrant, 2009). However, proboscideans also proved to be great travellers, and a flourishing diversity of proboscidean forms colonized most of the continents of the planet, including Europe, from where they have since completely disappeared. Nowadays, Loxodonta africana, L. cyclotis, and Elephas maximus are flagship species of the African and Asian faunas, but they only represent a minor part of the modern mammalian diversity. In contrast, their ancient relatives seemed to be relatively abundant in past ecosystems (Sanders et al., 2010), which raised a number of interesting, but challenging, questions relative to the structure and evolution of ancient megaherbivore communities (Calandra et al., 2008). Among proboscideans, deinotheres represent a special case. Their morphology clearly departs from that of other groups, notably in displaying distinctive downward curving lower tusks. Compared to their successful sister group the elephantiforms (i.e., all elephant-like proboscideans closely related to modern elephants; sensu Tassy, 1994), deinotheriids are often regarded as the poor sibling of the Proboscidea for showing a relatively low specific diversity and displaying a reduced morphological variability. In fact, many grey areas still exist regarding the evolution of this unique family. In their article, Gagliardi et al. (2021) revised the material of deinotheres recovered in the Miocene sands of the Swiss Jura Mountains. They described for the first time the material attributed to Prodeinotherium bavaricum and Deinotherium giganteum from the Delémont valley, and reported the presence of a third species, Deinotherium levius, from the locality of Charmoille in Ajoie. Based on comparisons made on specimens recovered from middle to the late Miocene localities, the authors discussed the potential link between the mode and tempo of deinothere dispersions and the evolution environmental and climatic conditions in Western and Eastern Europe during the Miocene. They also considered the evolution of ecological specializations in the group, especially with regard to size increase. Gagliardi et al. (2021) proposed to follow the two genera/five species concept (i.e., P. cuvieri, P. bavaricum, D. levius, D. giganteum, and D. proavum), which implies the co-existence of several deinothere species in Europe. The latter hypothesis contrasts with the recognition of a single African Deinotherium species (i.e., D. bozasi) in deposits dated from the late Miocene to the early Pleistocene (Sanders et al., 2010). Such a co-existence of European species was and still is debated; it was here questioned by both reviewers. However, as acknowledged by the authors, only an extensive revision of the material of all recognized species, in Europe and worldwide, will enable to shed more light on the deinothere morphological variability and specific diversity. There is no doubt that such a revision would have a profound impact on our view of the evolution of this enigmatic group.
References Asher, R. J., Bennett, N., & Lehmann, T. (2009). The new framework for understanding placental mammal evolution. BioEssays, 31(8), 853–864. doi: 10.1002/bies.200900053 Calandra, I., Göhlich, U. B., & Merceron, G. (2008). How could sympatric megaherbivores coexist? Example of niche partitioning within a proboscidean community from the Miocene of Europe. Naturwissenschaften, 95(9), 831–838. doi: 10.1007/s00114-008-0391-y Gagliardi, F., Maridet, O., & Becker, D. (2021). The record of Deinotheriidae from the Miocene of the Swiss Jura Mountains (Jura Canton, Switzerland). BioRxiv, 244061, ver. 4 peer-reviewed by PCI Paleo. doi: 10.1101/2020.08.10.244061 Gheerbrant, E. (2009). Paleocene emergence of elephant relatives and the rapid radiation of African ungulates. Proceedings of the National Academy of Sciences, 106(26), 10717–10721. doi: 10.1073/pnas.0900251106 Sanders, W. J., Gheerbrant, E., Harris, J. M., Saegusa, H., & Delmer, C. (2010). Proboscidea. In L. Werdelin & W. J. Sanders (Eds.), Cenozoic Mammals of Africa (pp. 161–251). Berkeley: University of California Press. doi: 10.1525/california/9780520257214.003.0015 Tassy, P. (1994). Origin and differentiation of the Elephantiformes (Mammalia, Proboscidea). Verhandlungen Naturwissenschaftlichen Vereins in Hamburg, 34, 73–94. | The record of Deinotheriidae from the Miocene of the Swiss Jura Mountains (Jura Canton, Switzerland) | Fanny Gagliardi, Olivier Maridet, Damien Becker | <p>The Miocene sands of the Swiss Jura Mountains, long exploited in quarries for the construction industry, have yielded abundant fossil remains of large mammals. Among Deinotheriidae (Proboscidea), two species, Prodeinotherium bavaricum and Deino... |  | Fossil record, Paleobiogeography, Taxonomy, Vertebrate paleontology | Lionel Hautier | 2020-08-11 10:17:38 | View | |
01 Oct 2021

Ammonoid taxonomy with supervised and unsupervised machine learning algorithmsFloe Foxon https://doi.org/10.31233/osf.io/ewkx9Performance of machine-learning approaches in identifying ammonoid species based on conch propertiesRecommended by Kenneth De Baets based on reviews by Jérémie Bardin and 1 anonymous reviewerThere are less and less experts on taxonomy of particular groups particularly among early career paleontologists and (paleo)biologists – this also includes ammonoid cephalopods. Techniques cannot replace this taxonomic expertise (Engel et al. 2021) but machine learning approaches can make taxonomy more efficient, reproducible as well as passing it over more sustainable. Initially ammonoid taxonomy was a black box with small differences sometimes sufficient to erect different species as well as really idiosyncratic groupings of superficially similar specimens (see De Baets et al. 2015 for a review). In the meantime, scientists have embraced more quantitative assessments of conch shape and morphology more generally (see Klug et al. 2015 for a more recent review). The approaches still rely on important but time-intensive collection work and seeing through daisy chains of more or less accessible papers and monographs without really knowing how these approaches perform (other than expert opinion). In addition, younger scientists are usually trained by more experienced scientists, but this practice is becoming more and more difficult which makes it difficult to resolve the taxonomic gap. This relates to the fact that less and less experienced researchers with this kind of expertise get employed as well as graduate students or postdocs choosing different research or job avenues after their initial training effectively leading to a leaky pipeline and taxonomic impediment. Robust taxonomy and stratigraphy is the basis for all other studies we do as paleontologists/paleobiologists so Foxon (2021) represents the first step to use supervised and unsupervised machine-learning approaches and test their efficiency on ammonoid conch properties. This pilot study demonstrates that machine learning approaches can be reasonably accurate (60-70%) in identifying ammonoid species (Foxon, 2021) – at least similar to that in other mollusk taxa (e.g., Klinkenbuß et al. 2020) - and might also be interesting to assist in cases where more traditional methods are not feasible. Novel approaches might even allow to further approve the accuracy as has been demonstrated for other research objects like pollen (Romero et al. 2020). Further applying of machine learning approaches on larger datasets and additional morphological features (e.g., suture line) are now necessary in order to test and improve the robustness of these approaches for ammonoids as well as test their performance more broadly within paleontology.
References De Baets K, Bert D, Hoffmann R, Monnet C, Yacobucci M, and Klug C (2015). Ammonoid intraspecific variability. In: Ammonoid Paleobiology: From anatomy to ecology. Ed. by Klug C, Korn D, De Baets K, Kruta I, and Mapes R. Vol. 43. Topics in Geobiology. Dordrecht: Springer, pp. 359–426. Engel MS, Ceríaco LMP, Daniel GM, Dellapé PM, Löbl I, Marinov M, Reis RE, Young MT, Dubois A, Agarwal I, Lehmann A. P, Alvarado M, Alvarez N, Andreone F, Araujo-Vieira K, Ascher JS, Baêta D, Baldo D, Bandeira SA, Barden P, Barrasso DA, Bendifallah L, Bockmann FA, Böhme W, Borkent A, Brandão CRF, Busack SD, Bybee SM, Channing A, Chatzimanolis S, Christenhusz MJM, Crisci JV, D’elía G, Da Costa LM, Davis SR, De Lucena CAS, Deuve T, Fernandes Elizalde S, Faivovich J, Farooq H, Ferguson AW, Gippoliti S, Gonçalves FMP, Gonzalez VH, Greenbaum E, Hinojosa-Díaz IA, Ineich I, Jiang J, Kahono S, Kury AB, Lucinda PHF, Lynch JD, Malécot V, Marques MP, Marris JWM, Mckellar RC, Mendes LF, Nihei SS, Nishikawa K, Ohler A, Orrico VGD, Ota H, Paiva J, Parrinha D, Pauwels OSG, Pereyra MO, Pestana LB, Pinheiro PDP, Prendini L, Prokop J, Rasmussen C, Rödel MO, Rodrigues MT, Rodríguez SM, Salatnaya H, Sampaio Í, Sánchez-García A, Shebl MA, Santos BS, Solórzano-Kraemer MM, Sousa ACA, Stoev P, Teta P, Trape JF, Dos Santos CVD, Vasudevan K, Vink CJ, Vogel G, Wagner P, Wappler T, Ware JL, Wedmann S, and Zacharie CK (2021). The taxonomic impediment: a shortage of taxonomists, not the lack of technical approaches. Zoological Journal of the Linnean Society 193, 381–387. doi: 10. 1093/zoolinnean/zlab072 Foxon F (2021). Ammonoid taxonomy with supervised and unsupervised machine learning algorithms. PaleorXiv ewkx9, ver. 3, peer-reviewed by PCI Paleo. doi: 10.31233/osf.io/ewkx9 Klinkenbuß D, Metz O, Reichert J, Hauffe T, Neubauer TA, Wesselingh FP, and Wilke T (2020). Performance of 3D morphological methods in the machine learning assisted classification of closely related fossil bivalve species of the genus Dreissena. Malacologia 63, 95. doi: 10.4002/040.063.0109 Klug C, Korn D, Landman NH, Tanabe K, De Baets K, and Naglik C (2015). Ammonoid conchs. In: Ammonoid Paleobiology: From anatomy to ecology. Ed. by Klug C, Korn D, De Baets K, Kruta I, and Mapes RH. Vol. 43. Dordrecht: Springer, pp. 3–24. Romero IC, Kong S, Fowlkes CC, Jaramillo C, Urban MA, Oboh-Ikuenobe F, D’Apolito C, and Punyasena SW (2020). Improving the taxonomy of fossil pollen using convolutional neural networks and superresolution microscopy. Proceedings of the National Academy of Sciences 117, 28496–28505. doi: 10.1073/pnas.2007324117 | Ammonoid taxonomy with supervised and unsupervised machine learning algorithms | Floe Foxon | <p>Ammonoid identification is crucial to biostratigraphy, systematic palaeontology, and evolutionary biology, but may prove difficult when shell features and sutures are poorly preserved. This necessitates novel approaches to ammonoid taxonomy. Th... |  | Invertebrate paleontology, Taxonomy | Kenneth De Baets | Jérémie Bardin | 2021-01-06 11:48:35 | View |
19 Sep 2023
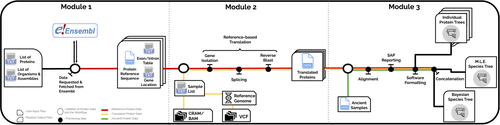
PaleoProPhyler: a reproducible pipeline for phylogenetic inference using ancient proteinsIoannis Patramanis, Jazmín Ramos-Madrigal, Enrico Cappellini, Fernando Racimo https://doi.org/10.1101/2022.12.12.519721An open-source pipeline to reconstruct phylogenies with paleoproteomic dataRecommended by Leslea Hlusko based on reviews by Katerina Douka and 2 anonymous reviewers based on reviews by Katerina Douka and 2 anonymous reviewers
One of the most recent technological advances in paleontology enables the characterization of ancient proteins, a new discipline known as palaeoproteomics (Ostrom et al., 2000; Warinner et al., 2022). Palaeoproteomics has superficial similarities with ancient DNA, as both work with ancient molecules, however the former focuses on peptides and the latter on nucleotides. While the study of ancient DNA is more established (e.g., Shapiro et al., 2019), palaeoproteomics is experiencing a rapid diversification of application, from deep time paleontology (e.g., Schroeter et al., 2022) to taxonomic identification of bone fragments (e.g., Douka et al., 2019), and determining genetic sex of ancient individuals (e.g., Lugli et al., 2022). However, as Patramanis et al. (2023) note in this manuscript, tools for analyzing protein sequence data are still in the informal stage, making the application of this methodology a challenge for many new-comers to the discipline, especially those with little bioinformatics expertise. In the spirit of democratizing the field of palaeoproteomics, Patramanis et al. (2023) developed an open-source pipeline, PaleoProPhyler released under a CC-BY license (https://github.com/johnpatramanis/Proteomic_Pipeline). Here, Patramanis et al. (2023) introduce their workflow designed to facilitate the phylogenetic analysis of ancient proteins. This pipeline is built on the methods from earlier studies probing the phylogenetic relationships of an extinct genus of rhinoceros Stephanorhinus (Cappellini et al., 2019), the large extinct ape Gigantopithecus (Welker et al., 2019), and Homo antecessor (Welker et al., 2020). PaleoProPhyler has three interacting modules that initialize, construct, and analyze an input dataset. The authors provide a demonstration of application, presenting a molecular hominid phyloproteomic tree. In order to run some of the analyses within the pipeline, the authors also generated the Hominid Palaeoproteomic Reference Dataset which includes 10,058 protein sequences per individual translated from publicly available whole genomes of extant hominids (orangutans, gorillas, chimpanzees, and humans) as well as some ancient genomes of Neanderthals and Denisovans. This valuable research resource is also publicly available, on Zenodo (Patramanis et al., 2022). Three reviewers reported positively about the development of this program, noting its importance in advancing the application of palaeoproteomics more broadly in paleontology. References Cappellini, E., Welker, F., Pandolfi, L., Ramos-Madrigal, J., Samodova, D., Rüther, P. L., Fotakis, A. K., Lyon, D., Moreno-Mayar, J. V., Bukhsianidze, M., Rakownikow Jersie-Christensen, R., Mackie, M., Ginolhac, A., Ferring, R., Tappen, M., Palkopoulou, E., Dickinson, M. R., Stafford, T. W., Chan, Y. L., … Willerslev, E. (2019). Early Pleistocene enamel proteome from Dmanisi resolves Stephanorhinus phylogeny. Nature, 574(7776), 103–107. https://doi.org/10.1038/s41586-019-1555-y Douka, K., Brown, S., Higham, T., Pääbo, S., Derevianko, A., and Shunkov, M. (2019). FINDER project: Collagen fingerprinting (ZooMS) for the identification of new human fossils. Antiquity, 93(367), e1. https://doi.org/10.15184/aqy.2019.3 Lugli, F., Nava, A., Sorrentino, R., Vazzana, A., Bortolini, E., Oxilia, G., Silvestrini, S., Nannini, N., Bondioli, L., Fewlass, H., Talamo, S., Bard, E., Mancini, L., Müller, W., Romandini, M., and Benazzi, S. (2022). Tracing the mobility of a Late Epigravettian (~ 13 ka) male infant from Grotte di Pradis (Northeastern Italian Prealps) at high-temporal resolution. Scientific Reports, 12(1), 8104. https://doi.org/10.1038/s41598-022-12193-6 Ostrom, P. H., Schall, M., Gandhi, H., Shen, T.-L., Hauschka, P. V., Strahler, J. R., and Gage, D. A. (2000). New strategies for characterizing ancient proteins using matrix-assisted laser desorption ionization mass spectrometry. Geochimica et Cosmochimica Acta, 64(6), 1043–1050. https://doi.org/10.1016/S0016-7037(99)00381-6 Patramanis, I., Ramos-Madrigal, J., Cappellini, E., and Racimo, F. (2022). Hominid Palaeoproteomic Reference Dataset (1.0.1) [dataset]. Zenodo. https://doi.org/10.5281/ZENODO.7333226 Patramanis, I., Ramos-Madrigal, J., Cappellini, E., and Racimo, F. (2023). PaleoProPhyler: A reproducible pipeline for phylogenetic inference using ancient proteins. BioRxiv, 519721, ver. 3 peer-reviewed by PCI Paleo. https://doi.org/10.1101/2022.12.12.519721 Schroeter, E. R., Cleland, T. P., and Schweitzer, M. H. (2022). Deep Time Paleoproteomics: Looking Forward. Journal of Proteome Research, 21(1), 9–19. https://doi.org/10.1021/acs.jproteome.1c00755 Shapiro, B., Barlow, A., Heintzman, P. D., Hofreiter, M., Paijmans, J. L. A., and Soares, A. E. R. (Eds.). (2019). Ancient DNA: Methods and Protocols (2nd ed., Vol. 1963). Humana, New York. https://doi.org/10.1007/978-1-4939-9176-1 Warinner, C., Korzow Richter, K., and Collins, M. J. (2022). Paleoproteomics. Chemical Reviews, 122(16), 13401–13446. https://doi.org/10.1021/acs.chemrev.1c00703 Welker, F., Ramos-Madrigal, J., Gutenbrunner, P., Mackie, M., Tiwary, S., Rakownikow Jersie-Christensen, R., Chiva, C., Dickinson, M. R., Kuhlwilm, M., De Manuel, M., Gelabert, P., Martinón-Torres, M., Margvelashvili, A., Arsuaga, J. L., Carbonell, E., Marques-Bonet, T., Penkman, K., Sabidó, E., Cox, J., … Cappellini, E. (2020). The dental proteome of Homo antecessor. Nature, 580(7802), 235–238. https://doi.org/10.1038/s41586-020-2153-8 Welker, F., Ramos-Madrigal, J., Kuhlwilm, M., Liao, W., Gutenbrunner, P., De Manuel, M., Samodova, D., Mackie, M., Allentoft, M. E., Bacon, A.-M., Collins, M. J., Cox, J., Lalueza-Fox, C., Olsen, J. V., Demeter, F., Wang, W., Marques-Bonet, T., and Cappellini, E. (2019). Enamel proteome shows that Gigantopithecus was an early diverging pongine. Nature, 576(7786), 262–265. https://doi.org/10.1038/s41586-019-1728-8 | PaleoProPhyler: a reproducible pipeline for phylogenetic inference using ancient proteins | Ioannis Patramanis, Jazmín Ramos-Madrigal, Enrico Cappellini, Fernando Racimo | <p>Ancient proteins from fossilized or semi-fossilized remains can yield phylogenetic information at broad temporal horizons, in some cases even millions of years into the past. In recent years, peptides extracted from archaic hominins and long-ex... |  | Evolutionary biology, Paleoanthropology, Paleogenetics & Ancient DNA, Phylogenetics | Leslea Hlusko | 2023-02-24 13:40:12 | View |
MANAGING BOARD
Jérémy Anquetin
Faysal Bibi
Guillaume Billet
Andrew A. Farke
Franck Guy
Leslea J. Hlusko
Melanie Hopkins
Cynthia V. Looy
Jesús Marugán-Lobón
Ilaria Mazzini
P. David Polly
Caroline A.E. Strömberg










