Latest recommendations

| Id▲ | Title * | Authors * | Abstract * | Picture * | Thematic fields * | Recommender | Reviewers | Submission date | |
|---|---|---|---|---|---|---|---|---|---|
22 Sep 2018
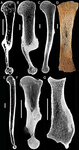
Palaeobiological inferences based on long bone epiphyseal and diaphyseal structure - the forelimb of xenarthrans (Mammalia)Eli Amson & John A. Nyakatura https://doi.org/10.1101/318121Inferences on the lifestyle of fossil xenarthrans based on limb long bone inner structureRecommended by Alexandra Houssaye based on reviews by Andrew Pitsillides and 1 anonymous reviewerBone inner structure bears a strong functional signal and can be used in paleontology to make inferences about the ecology of fossil forms. The increasing use of microtomography enables to analyze both cortical and trabecular features in three dimensions, and thus in long bones to investigate the diaphyseal and epiphyseal structures. Moreover, this can now be done through quantitative, and not only qualitative analyses. Studies focusing on the diaphyseal inner structure (cortical bone and sometimes also spongious bone) of long bones are rather numerous, but essentially based on 2D sections. It is only recently that analyses of the whole diaphyseal structure have been investigated. Studies on the trabecular architecture are much rarer. Amson & Nyakatura (2018) propose a comparative quantitative analysis combining parameters of the epiphyseal trabecular architecture and of the diaphyseal structure, using phylogenetically informed discriminant analyses, and with the aim of inferring the lifestyle of extinct taxa. The group of interest is xenarthrans, one of the four major extant clades of placental mammals. Xenarthrans exhibit different lifestyles, from fully terrestrial to arboreal, and show various degrees of fossoriality. The authors analyzed forelimb long bones of some fossil sloths and made comparisons with several species of extant xenarthrans. The aim was notably to discuss the degree of arboreality and fossoriality of these fossil forms. This study is among the first ones to conjointly analyze both diaphyseal and trabecular parameters to characterize lifestyles, and the first one outside of primates. No fossil form could undoubtedly be assigned to one lifestyle exhibited by extant xenarthrans, though some previous ecological hypotheses could be corroborated. This study also raised some technical challenges, linked to the sample and to the parameters studied, and thus constitutes a great step, from which to go further. References Amson, E., & Nyakatura, J. A. (2018). Palaeobiological inferences based on long bone epiphyseal and diaphyseal structure - the forelimb of xenarthrans (Mammalia). bioRxiv, 318121, ver. 5 peer-reviewed and recommended by PCI Paleo. doi: 10.1101/318121 | Palaeobiological inferences based on long bone epiphyseal and diaphyseal structure - the forelimb of xenarthrans (Mammalia) | Eli Amson & John A. Nyakatura | <p>Trabecular architecture (i.e., the main orientation of the bone trabeculae, their number, mean thickness, spacing, etc.) has been shown experimentally to adapt with great accuracy and sensitivity to the loadings applied to the bone during life.... |  | Biomechanics & Functional morphology, Comparative anatomy, Evolutionary biology, Histology, Methods, Morphological evolution, Paleobiology, Vertebrate paleontology | Alexandra Houssaye | 2018-05-14 08:35:20 | View | |
16 Oct 2019

What do ossification sequences tell us about the origin of extant amphibians?Michel Laurin, Océane Lapauze, David Marjanović https://doi.org/10.1101/352609The origins of LissamphibiaRecommended by Robert Asher based on reviews by Jennifer Olori and 2 anonymous reviewersAmong living vertebrates, there is broad consensus that living tetrapods consist of amphibians and amniotes. Crown clade Lissamphibia contains frogs (Anura), salamanders (Urodela) and caecilians (Gymnophiona); Amniota contains Sauropsida (reptiles including birds) and Synapsida (mammals). Within Lissamphibia, most studies place frogs and salamanders in a clade together to the exclusion of caecilians (see Pyron & Wiens 2011). Among fossils, there are a number of amphibian and amphibian-like taxa generally placed in Temnospondyli and Lepospondyli. In contrast to the tree of living tetrapods, affinities of these fossils to some or all of the three extant lissamphibian groups have proven to be much harder to resolve. For example, temnospondyls might be stem tetrapods and lissamphibians a derived group of lepospondyls; alternatively, temnospondyls might be closer to the clade of frogs and salamanders, and lepospondyls to caecilians (compare Laurin et al. 2019: fig. 1d vs. 1f). Here, in order to assess which of these and other mutually exclusive topologies is optimal, Laurin et al. (2019) extract phylogenetic information from developmental sequences, in particular ossification. Several major differences in ossification are known to distinguish vertebrate clades. For example, due to their short intrauterine development and need to climb from the reproductive tract into the pouch, marsupial mammals famously accelerate ossification of their facial skeleton and forelimb; in contrast to placentals, newborn marsupials can climb, smell & suck before they have much in the way of lungs, kidneys, or hindlimbs (Smith 2001). Divergences among living and fossil amphibian groups are likely pre-Triassic (San Mauro 2010; Pyron 2011), much older than a Jurassic split between marsupials and placentals (Tarver et al. 2016), and the quality of the fossil record generally decreases with ever-older divergences. Nonetheless, there are a number of well-preserved examples of "amphibian"-grade tetrapods representing distinct ontogenetic stages (Schoch 2003, 2004; Schoch and Witzmann 2009; Olori 2013; Werneburg 2018; among others), all amenable to analysis of ossification sequences. Putting together a phylogenetic dataset based on ossification sequences is not trivial; sequences are not static features apparent on individual specimens. Rather, one needs multiple specimens representing discrete developmental stages for each taxon to be compared, meaning that sequences are usually available for only a few characters. Laurin et al. (2019) have nonetheless put together the most exhaustive matrix of tetrapod sequences so far, with taxon coverage ranging from 62 genera for appendicular characters to 107 for one of their cranial datasets, each sampling between 4-8 characters (Laurin et al. 2019: table 1). The small number of characters means that simply applying an optimality criterion (such as parsimony) is unlikely to resolve most nodes; treespace is too flat to be able to offer optimal peaks up which a search algorithm might climb. However, Laurin et al. (2019) were able to test each of the main competing hypotheses, defined a priori as a branching topology, given their ossification sequence dataset and a likelihood optimality criterion. Their most consistent result comes from their cranial ossification sequences and supports their "LH", or lepospondyl hypothesis (Laurin et al. 2019: fig. 1d). That is, relative to extinct, "amphibian"-grade taxa, Lissamphibia is monophyletic and nested within lepospondyls. Compared to mammals and birds (including dinosaurs), crown amphibian branches of the Tree of Life are exceptionally old. Each lissamphibian clade likely had diverged during Permian times (Marjanovic & Laurin 2008) and the crown group itself may even date to the Carboniferous (Pyron 2011). In contrast to mammoths and moas, no ancient DNA or collagen sequences are going to be available from >300 million-year-old fossils like the lepospondyl *Hyloplesion* (Olori 2013), although recently published methods for incorporating genomic signal from extant taxa (Beck & Baillie 2018; Asher et al. 2019) into studies of fossils could also be applied to these ancient divergences among amphibian-grade tetrapods. Ossification sequences represent another important, additional source of data with which to test the conclusion of Laurin et al. (2019) that monophyletic Lissamphibians shared a common ancestor with lepospondyls, among other hypotheses. **References** Asher, R. J., Smith, M. R., Rankin, A., & Emry, R. J. (2019). Congruence, fossils and the evolutionary tree of rodents and lagomorphs. Royal Society Open Science, 6(7), 190387. doi: [ 10.1098/rsos.190387 ](https://dx.doi.org/ 10.1098/rsos.190387 ) Beck, R. M. D., & Baillie, C. (2018). Improvements in the fossil record may largely resolve current conflicts between morphological and molecular estimates of mammal phylogeny. Proceedings of the Royal Society B: Biological Sciences, 285(1893), 20181632. doi: [ 10.1098/rspb.2018.1632](https://dx.doi.org/ 10.1098/rspb.2018.1632) Laurin, M., Lapauze, O., & Marjanović, D. (2019). What do ossification sequences tell us about the origin of extant amphibians? BioRxiv, 352609, ver. 4 peer-reviewed by PCI Paleo. doi: [ 10.1101/352609](https://dx.doi.org/ 10.1101/352609) Marjanović, D., & Laurin, M. (2008). Assessing confidence intervals for stratigraphic ranges of higher taxa: the case of Lissamphibia. Acta Palaeontologica Polonica, 53(3), 413–432. doi: [ 10.4202/app.2008.0305](https://dx.doi.org/ 10.4202/app.2008.0305) Olori, J. C. (2013). Ontogenetic sequence reconstruction and sequence polymorphism in extinct taxa: an example using early tetrapods (Tetrapoda: Lepospondyli). Paleobiology, 39(3), 400–428. doi: [ 10.1666/12031](https://dx.doi.org/ 10.1666/12031) Pyron, R. A. (2011). Divergence time estimation using fossils as terminal taxa and the origins of Lissamphibia. Systematic Biology, 60(4), 466–481. doi: [ 10.1093/sysbio/syr047](https://dx.doi.org/ 10.1093/sysbio/syr047) Pyron, R. A., & Wiens, J. J. (2011). A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Molecular Phylogenetics and Evolution, 61(2), 543–583. doi: [ 10.1016/j.ympev.2011.06.012](https://dx.doi.org/ 10.1016/j.ympev.2011.06.012) San Mauro, D. (2010). A multilocus timescale for the origin of extant amphibians. Molecular Phylogenetics and Evolution, 56(2), 554–561. doi: [ 10.1016/j.ympev.2010.04.019](https://dx.doi.org/ 10.1016/j.ympev.2010.04.019) Schoch, R. R. (2003). Early larval ontogeny of the Permo-Carboniferous temnospondyl *Sclerocephalus*. Palaeontology, 46(5), 1055–1072. doi: [ 10.1111/1475-4983.00333](https://dx.doi.org/ 10.1111/1475-4983.00333) Schoch, R. R. (2004). Skeleton formation in the Branchiosauridae: a case study in comparing ontogenetic trajectories. Journal of Vertebrate Paleontology, 24(2), 309–319. doi: [ 10.1671/1950](https://dx.doi.org/ 10.1671/1950) Schoch, R. R., & Witzmann, F. (2009). Osteology and relationships of the temnospondyl genus *Sclerocephalus*. Zoological Journal of the Linnean Society, 157(1), 135–168. doi: [ 10.1111/j.1096-3642.2009.00535.x](https://dx.doi.org/ 10.1111/j.1096-3642.2009.00535.x) Smith, K. K. (2001). Heterochrony revisited: the evolution of developmental sequences. Biological Journal of the Linnean Society, 73(2), 169–186. doi: [ 10.1111/j.1095-8312.2001.tb01355.x](https://dx.doi.org/ 10.1111/j.1095-8312.2001.tb01355.x) Tarver, J. E., dos Reis, M., Mirarab, S., Moran, R. J., Parker, S., O’Reilly, J. E., & Pisani, D. (2016). The interrelationships of placental mammals and the limits of phylogenetic inference. Genome Biology and Evolution, 8(2), 330–344. doi: [ 10.1093/gbe/evv261](https://dx.doi.org/ 10.1093/gbe/evv261) Werneburg, R. (2018). Earliest “nursery ground” of temnospondyl amphibians in the Permian. Semana, 32, 3–42. | What do ossification sequences tell us about the origin of extant amphibians? | Michel Laurin, Océane Lapauze, David Marjanović | <p>The origin of extant amphibians has been studied using several sources of data and methods, including phylogenetic analyses of morphological data, molecular dating, stratigraphic data, and integration of ossification sequence data, but a consen... |  | Evo-Devo, Phylogenetics, Systematics, Vertebrate paleontology | Robert Asher | 2018-06-22 08:21:31 | View | |
30 Oct 2019

The Morrison Formation Sauropod Consensus: A freely accessible online spreadsheet of collected sauropod specimens, their housing institutions, contents, references, localities, and other potentially useful informationEmanuel Tschopp, John A. Whitlock, D. Cary Woodruff, John R. Foster, Roberto Lei, Simone Giovanardi https://doi.org/10.31233/osf.io/ydvraSauropods under one (very high) roofRecommended by Jordan Mallon based on reviews by Kenneth Carpenter and Femke HolwerdaFossils get around. Any one fossil locality might be sampled by several collectors from as many institutions around the world. Alternatively, a single collector might heavily sample a site, and sell or trade parts of their collection to other institutions, scattering the fossils far and wide. These practices have the advantage of making fossils from any one locality available to researchers across the globe. However, they also have the disadvantage that, in order to systematically survey any one species, a researcher must follow innumerable trails of breadcrumb to get to where the relevant materials are held. This is true of many famous fossil localities, such as the Eocene Green River Formation in the USA, the Cretaceous Kem Kem beds of Morocco, or the Devonian Miguasha cliffs of Canada. It is especially true of the Upper Jurassic deposits of the Morrison Formation in the western USA, which have yielded an impressive assemblage of megaherbivorous sauropod dinosaurs over the last 150 years. Today, these bones are to be found in museums not just in the USA, but also in Canada, Argentina, Japan, Australia, Malaysia, South Africa, and throughout Europe. Trawling museum databases in search of sauropod material from the Morrison Formation can therefore be a daunting task, never mind traveling the globe to actually study them. A new paper by Tschopp et al. (2019) seeks to ease the burden on sauropod researchers by introducing a database of Morrison Formation sauropods, consisting of over 3000 specimens housed in nearly 40 institutions around the world. The authors are themselves sauropod workers and, having suffered first-hand the plight of studying material from the Morrison Formation, came up with a solution to the problem of keeping track of it all. The database is founded largely on material personally seen by the authors, supplemented by information from the literature and museum catalogs. The database further provides information on bone representation, ontogeny, locality details, and fine-scale stratigraphy, among other fields. Like any database, it is a living document that will continue to grow as new finds are made. Tschopp et al. (2019) have wisely chosen to allow others to contribute to the listing, but changes must first be vetted for accuracy. This product represents 10 years of work, and I have little doubt that it will be well-received by those of us who work on dinosaurs. Speaking personally, my PhD research on megaherbivorous dinosaurs from the Dinosaur Park Formation of Canada led me to institutions in Canada, the USA, and the UK, and further stops to Spain and Argentina would have been beneficial, if affordable. Planning for this work would have been greatly assisted by a database like the one provided us by Tschopp et al. (2019). Many a future graduate student will undoubtedly owe them a debt of gratitude. References Tschopp, E., Whitlock, J. A., Woodruff, D. C., Foster, J. R., Lei, R., & Giovanardi, S. (2019). The Morrison Formation Sauropod Consensus: A freely accessible online spreadsheet of collected sauropod specimens, their housing institutions, contents, references, localities, and other potentially useful information. PaleorXiv, version 3, peer-reviewed by PCI Paleo. doi: 10.31233/osf.io/ydvra | The Morrison Formation Sauropod Consensus: A freely accessible online spreadsheet of collected sauropod specimens, their housing institutions, contents, references, localities, and other potentially useful information | Emanuel Tschopp, John A. Whitlock, D. Cary Woodruff, John R. Foster, Roberto Lei, Simone Giovanardi | <p>The Morrison Formation has been explored for dinosaurs for more than 150 years, in particular for large sauropod skeletons to be mounted in museum exhibits around the world. Several long-term campaigns to the Jurassic West of the United States ... |  | Fossil record, Methods, Paleobiodiversity, Taxonomy, Vertebrate paleontology | Jordan Mallon | 2019-07-19 16:13:45 | View | |
27 Jan 2020

A simple generative model of trilobite segmentation and growthMelanie J Hopkins https://doi.org/10.31233/osf.io/zt642Deep insights into trilobite developmentRecommended by Christian Klug based on reviews by Kenneth De Baets and Lukas LaiblTrilobites are arthropods that became extinct at the greatest marine mass extinction over 250 Ma ago. Because of their often bizarre forms, their great diversity and disparity of shapes, they have attracted the interest of researchers and laypersons alike. Due to their calcified exoskeleton, their remains are quite abundant in many marine strata. One particularly interesting aspect, however, is the fossilization of various molting stages. This allows the reconstruction of both juvenile strategies (lecitotrophic versus planktotrophic) and the entire life history of at least some well-documented taxa (e.g., Hughes 2003, 2007; Laibl 2017). For example, life history of trilobites is, based on certain morphological changes, classically subdivided in the three phases protaspis (hatchling, one dorsal shield with few segments with no articulation between), meraspis (juvenile, two and more shields connected by articulations) and holaspis (when the terminal number of thoracic segments is reached). At most molting events, a new skeletal element is added (only in the holaspis, the number of thoracic segments does not change). Nevertheless, many trilobites are known mainly from late meraspid and holaspid stages, because the dorsal shields of the first ontogenetic stages are usually very small and thus often either dissolved or overlooked. An improved understanding of trilobite ontogeny could thus help filling in these gaps in fossil preservation and subsequently, to better understand evolutionary pathways. This is where this paper comes in. In a very clever approach, the New-York-based researcher Melanie Hopkins modeled the growth of these segmented animals (Hopkins 2020). Previous growth models of invertebrates focused on, e.g., mollusks, whose shells grow by accretion. Modelling arthropod ontogeny represented a challenge, which is now overcome by Hopkins' brilliant paper. Her generative growth model is based on empirical data of Aulacopleura koninckii (Barrande, 1846). Hong et al. (2014) and Hughes et al. (2017) documented the ontogeny of this 429 Ma old trilobite species in great detail. In the Silurian of the Barrandian region (Czech Republic), this species is locally very common and all growth stages are well known. I could imagine that the paper of Hughes et al. (2017) planted the seed into Melanie Hopkins’ mind to approach trilobite development in general in a quantitative way with a mathematical approach comparable to the mollusk-research by, e.g., David Raup (1961, 1966) and George McGhee (2015). Hopkins’ growth model requires “a minimum of nine parameters […] to model basic trilobite growth and segmentation, and three additional parameters […] to allow a transition to a new growth gradient for the trunk region during ontogeny” (Hopkins 2020: p. 21). It is now possible to play with parameters such as protaspid size, segment dimensions, segment numbers, etc., in order to estimate changes in body size or morphology. Furthermore, the model could be applied to similarly organized arthropod exoskeletons like many early Cambrian arthropods (e.g., marellomorphs) or even crustaceans (e.g., conchostracans or copepods). Of great interest could also be to assess influences of environmental changes on arthropod ontogeny. Also, her work will help to reconstruct unknown developmental information missing from trilobite species (and possibly other arthropods) and also to explore their morphospace. References Barrande, J. (1846). Notice préliminaire sur le système Silurien et les trilobites de Bohême. Leipzig: Hirschfield.
Hong, P. S., Hughes, N. C., & Sheets, H. D. (2014). Size, shape, and systematics of the Silurian trilobite Aulacopleura koninckii. Journal of Paleontology, 88(6), 1120–1138. doi: 10.1666/13-142 | A simple generative model of trilobite segmentation and growth | Melanie J Hopkins | <p>Generative growth models have been the basis for numerous studies of morphological diversity and evolution. Most work has focused on modeling accretionary growth systems, with much less attention to discrete growth systems. Generative growth mo... |  | Evo-Devo, Evolutionary biology, Invertebrate paleontology, Paleobiology | Christian Klug | 2019-10-06 00:27:25 | View | |
27 May 2020

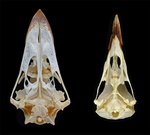
The last surviving Thalassochelydia—A new turtle cranium from the Early Cretaceous of the Purbeck Group (Dorset, UK)Jérémy Anquetin, Charlotte André https://doi.org/10.31233/osf.io/7pa5cA recommendation of: The last surviving Thalassochelydia—A new turtle cranium from the Early Cretaceous of the Purbeck Group (Dorset, UK)Recommended by Hans-Dieter Sues based on reviews by Igor Danilov and Serjoscha EversStem- and crown-group turtles have a rich and varied fossil record dating back to the Triassic Period. By far the most common remains of these peculiar reptiles are their bony shells and fragments of shells. Furthermore, if historical specimens preserved skulls the preparation techniques at that time were inadequate for elucidating details of the cranial structure. Thus, it comes as no surprise that most of the early research on turtles focused on the structure of the shell with little attention paid to other parts of the skeleton. Starting in the 1960s, this changed as researchers realized that there is considerable variation in the structure of turtle shells even within species and that new methods of fossil preparation, especially chemical methods, could reveal a wealth of phylogenetically important features in the structure of the skulls of turtles. The principal worker was Eugene S. Gaffney of the American Museum of Natural History (New York) who in a series of exquisitely illustrated monographs revolutionized our understanding of turtle osteology and phylogeny. Over the last decade or so, a new generation of researchers has further refined the phylogenetic framework for turtles and continued the work by Gaffney. One of the specialists from this new generation is Jérémy Anquetin who, with a number of colleagues, has revised many of the Jurassic-age stem-turtles that existed in coastal marine settings in what is now Europe. Collections in France, Germany, Switzerland, and the UK house numerous specimens of these forms, which attracted the interest of researchers as early as the first decades of the nineteenth century. Despite this long history, however, the diversity and interrelationships of these marine taxa remained poorly understood. In the present study, Anquetin and his colleague Charlotte André extend the fossil record of these stem-turtles, recently hypothesized as a clade Thalassochelydia, into the Early Cretaceous (Anquetin & André 2020). They present an excellent anatomical account on a well-preserved cranium from the Purbeck Formation of Dorset (England) that can be referred to Thalassochelydia and augments our knowledge of the cranial morphology of this clade. Anquetin & André (2020) make a good case that this specimen belongs to the same taxon as shell material long ago described as Hylaeochelys belli. References Anquetin, J., & André, C. (2020). The last surviving Thalassochelydia—A new turtle cranium from the Early Cretaceous of the Purbeck Group (Dorset, UK). PaleorXiv, 7pa5c, version 3, peer-reviewed by PCI Paleo. doi: 10.31233/osf.io/7pa5c | The last surviving Thalassochelydia—A new turtle cranium from the Early Cretaceous of the Purbeck Group (Dorset, UK) | Jérémy Anquetin, Charlotte André | <p>**Background.** The mostly Berriasian (Early Cretaceous) Purbeck Group of southern England has produced a rich turtle fauna dominated by the freshwater paracryptodires *Pleurosternon bullockii* and *Dorsetochelys typocardium*. Each of these spe... |  | Comparative anatomy, Paleoecology, Phylogenetics, Systematics, Vertebrate paleontology | Hans-Dieter Sues | 2020-01-30 10:37:07 | View | |
20 Oct 2020
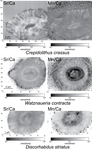
Evidence of high Sr/Ca in a Middle Jurassic murolith coccolith speciesBaptiste Suchéras-Marx, Fabienne Giraud, Alexandre Simionovici, Rémi Tucoulou, Isabelle Daniel https://doi.org/10.31233/osf.io/dcfuqNew results and challenges in Sr/Ca studies on Jurassic coccolithophoridsRecommended by Antonino Briguglio based on reviews by Kenneth De Baets and 1 anonymous reviewerThis interesting publication by Suchéras-Marx et al. (2020) highlights peculiar aspects of geochemistry in nannofossils, specifically coccolithophorids. One of the main application of geochemistry on fossil shells is to get hints on the physiology of such extinct taxa. Here, the authors try to get information on the calcification mechanism and processes in Jurassic coccoliths. Coccoliths build a test made of calcium carbonate and one of the most common geochemical proxies used for this fossil group is the Sr/Ca ratio. This isotopic ratio has good chances to be successfully used as a robust proxy for paleoenvironmental reconstruction, but, concerning Jurassic coccoliths things seem to be not straightforward. The authors managed to compare the isotopic value of Sr/Ca measured on Jurassic coccoliths from different taxonomic groups: the murolith Crepidolithus crassus and the placoliths Watznaueria contracta and Discorhabdus striatus. The results they got clearly show that the Sr/Ca ratio cannot be used as a universal proxy because these species exhibit very different values despite coming from the same stratigraphic level and having undergone minimal diagenetic modification. Data seem to point to a Sr/Ca ratio up to 10 times higher in the murolith species than in the placolith taxa (Suchéras-Marx et al., 2020). One of the explanation given here takes advantage of modern coccolith data and hints to specific polysaccharides that would control the growth of the long R unit in the murolith species. As always, there is plenty of space for additional research, possibly on modern taxa, to sort out the scientific questions that arise from this work. References Suchéras-Marx, B., Giraud, F., Simionovici, A., Tucoulou, R., & Daniel, I. (2020). Evidence of high Sr/Ca in a Middle Jurassic murolith coccolith species. PaleorXiv, dcfuq, version 7, peer-reviewed by PCI Paleo. doi: 10.31233/osf.io/dcfuq | Evidence of high Sr/Ca in a Middle Jurassic murolith coccolith species | Baptiste Suchéras-Marx, Fabienne Giraud, Alexandre Simionovici, Rémi Tucoulou, Isabelle Daniel | <p>Paleoceanographical reconstructions are often based on microfossil geochemical analyses. Coccoliths are the most ancient, abundant and continuous record of pelagic photic zone calcite producer organisms. Hence, they could be valuable substrates... |  | Microfossils, Micropaleontology, Nanofossils | Antonino Briguglio | 2020-05-18 16:11:35 | View | |
14 Apr 2021
The impact of allometry on vomer shape and its implications for the taxonomy and cranial kinesis of crown-group birdsOlivia Plateau, Christian Foth https://doi.org/10.1101/2020.07.02.184101Vomers aren't so different in crown group birds when considering allometric effectsRecommended by Andrew Farke based on reviews by Sergio Martínez Nebreda and Roland SookiasToday’s birds are divided into two deeply divergent and historically well-documented groups: Palaeognathae and Neognathae. Palaeognaths include both the flight-capable tinamous as well as the flightless ratites (ostriches, rheas, kiwis, cassowaries, and kin). Neognaths include all other modern birds, ranging from sparrows to penguins to hummingbirds. The clade names refer to the anatomy of the palate, with the “old jaws” (palaeognaths) originally thought to more closely resemble an ancestral reptilian condition and the “new jaws” (neognaths) showing a uniquely modified bony configuration. This particularly manifests in the pterygoid-palatine complex (PPC) in the palate, formed from pairs of pterygoids and palatines alongside a single midline vomer. In palaeognaths, the vomer is comparatively large and the pterygoid and palatine are relatively tightly connected. The PPC is more mobile in neognaths, with a variably shaped vomer, which is sometimes even absent. Although both groups of birds show cranial kinesis, neognaths exhibit a much more pronounced degree of kinesis versus palaeognaths, due in part to the tighter nature of the palaeognath pterygoid/palatine interfaces. A previous paper (Hu et al. 2019) used 3D geometric morphometrics to compare the shape of the vomer across neognaths and palaeognaths. Among other findings, this work suggested that each clade had a distinct vomer morphology, with palaeognaths more similar to the ancestral condition (i.e., that of non-avian dinosaurs). This observation was extended to support inferences of limited vs. less limited cranial kinesis in various extinct species, based in part on observations of vomer shape. A new preprint by Plateau and Foth (2021) presents a reanalysis of Hu et al.’s data, specifically focusing on allometric effects. In short, the new analysis looks at how size correlates (or doesn't correlate) with vomer shape. Plateau and Foth (2021) found that when size effects are included, differences between palaeognaths and neognaths are less than the “raw” (uncorrected) shape data suggest. It is much harder to tell bird groups apart! Certainly, there are still some general differences, but some separations in morphospace close up when allometry—the interrelationship between shape and size—is considered. Plateau and Foth (2021) use this finding to suggest that 1) vomer shape alone is not a completely reliable proxy for inferring the phylogenetic affinities of a particular bird; and 2) the vomer is only one small component of the cranial kinetic system, and thus its shape is of limited utility for inferring cranial kinesis capabilities when considered independently from the rest of the relevant skull bones.
References Hu, H., Sansalone, G., Wroe, S., McDonald, P. G., O’Connor, J. K., Li, Z., Xu, X., & Zhou, Z. (2019). Evolution of the vomer and its implications for cranial kinesis in Paraves. Proceedings of the National Academy of Sciences, 116(39), 19571–19578. doi: 10.1073/pnas.1907754116 | The impact of allometry on vomer shape and its implications for the taxonomy and cranial kinesis of crown-group birds | Olivia Plateau, Christian Foth | <p>Crown birds are subdivided into two main groups, Palaeognathae and Neognathae, that can be distinguished, among others, by the organization of the bones in their pterygoid-palatine complex (PPC). Shape variation to the vomer, which is the most ... |  | Comparative anatomy, Evolutionary biology, Macroevolution, Morphological evolution, Morphometrics, Taxonomy | Andrew Farke | 2020-07-03 14:16:48 | View | |
23 Apr 2021

The record of Deinotheriidae from the Miocene of the Swiss Jura Mountains (Jura Canton, Switzerland)Fanny Gagliardi, Olivier Maridet, Damien Becker https://doi.org/10.1101/2020.08.10.244061The fossil record of deinotheres in the Jura Mountains and the specific diversity of European deinotheriidsRecommended by Lionel Hautier based on reviews by Martin Pickford and 1 anonymous reviewerProboscideans belong to the Afrotheria, a superorder of mammals with an African origin, which was recently recognized based on molecular data (see review in Asher et al., 2009). The fossil record of Proboscidea is well documented and shows that an important part of their evolutionary history took place in Africa, with their representatives inhabiting the continent for at least 60 million years (Gheerbrant, 2009). However, proboscideans also proved to be great travellers, and a flourishing diversity of proboscidean forms colonized most of the continents of the planet, including Europe, from where they have since completely disappeared. Nowadays, Loxodonta africana, L. cyclotis, and Elephas maximus are flagship species of the African and Asian faunas, but they only represent a minor part of the modern mammalian diversity. In contrast, their ancient relatives seemed to be relatively abundant in past ecosystems (Sanders et al., 2010), which raised a number of interesting, but challenging, questions relative to the structure and evolution of ancient megaherbivore communities (Calandra et al., 2008). Among proboscideans, deinotheres represent a special case. Their morphology clearly departs from that of other groups, notably in displaying distinctive downward curving lower tusks. Compared to their successful sister group the elephantiforms (i.e., all elephant-like proboscideans closely related to modern elephants; sensu Tassy, 1994), deinotheriids are often regarded as the poor sibling of the Proboscidea for showing a relatively low specific diversity and displaying a reduced morphological variability. In fact, many grey areas still exist regarding the evolution of this unique family. In their article, Gagliardi et al. (2021) revised the material of deinotheres recovered in the Miocene sands of the Swiss Jura Mountains. They described for the first time the material attributed to Prodeinotherium bavaricum and Deinotherium giganteum from the Delémont valley, and reported the presence of a third species, Deinotherium levius, from the locality of Charmoille in Ajoie. Based on comparisons made on specimens recovered from middle to the late Miocene localities, the authors discussed the potential link between the mode and tempo of deinothere dispersions and the evolution environmental and climatic conditions in Western and Eastern Europe during the Miocene. They also considered the evolution of ecological specializations in the group, especially with regard to size increase. Gagliardi et al. (2021) proposed to follow the two genera/five species concept (i.e., P. cuvieri, P. bavaricum, D. levius, D. giganteum, and D. proavum), which implies the co-existence of several deinothere species in Europe. The latter hypothesis contrasts with the recognition of a single African Deinotherium species (i.e., D. bozasi) in deposits dated from the late Miocene to the early Pleistocene (Sanders et al., 2010). Such a co-existence of European species was and still is debated; it was here questioned by both reviewers. However, as acknowledged by the authors, only an extensive revision of the material of all recognized species, in Europe and worldwide, will enable to shed more light on the deinothere morphological variability and specific diversity. There is no doubt that such a revision would have a profound impact on our view of the evolution of this enigmatic group.
References Asher, R. J., Bennett, N., & Lehmann, T. (2009). The new framework for understanding placental mammal evolution. BioEssays, 31(8), 853–864. doi: 10.1002/bies.200900053 Calandra, I., Göhlich, U. B., & Merceron, G. (2008). How could sympatric megaherbivores coexist? Example of niche partitioning within a proboscidean community from the Miocene of Europe. Naturwissenschaften, 95(9), 831–838. doi: 10.1007/s00114-008-0391-y Gagliardi, F., Maridet, O., & Becker, D. (2021). The record of Deinotheriidae from the Miocene of the Swiss Jura Mountains (Jura Canton, Switzerland). BioRxiv, 244061, ver. 4 peer-reviewed by PCI Paleo. doi: 10.1101/2020.08.10.244061 Gheerbrant, E. (2009). Paleocene emergence of elephant relatives and the rapid radiation of African ungulates. Proceedings of the National Academy of Sciences, 106(26), 10717–10721. doi: 10.1073/pnas.0900251106 Sanders, W. J., Gheerbrant, E., Harris, J. M., Saegusa, H., & Delmer, C. (2010). Proboscidea. In L. Werdelin & W. J. Sanders (Eds.), Cenozoic Mammals of Africa (pp. 161–251). Berkeley: University of California Press. doi: 10.1525/california/9780520257214.003.0015 Tassy, P. (1994). Origin and differentiation of the Elephantiformes (Mammalia, Proboscidea). Verhandlungen Naturwissenschaftlichen Vereins in Hamburg, 34, 73–94. | The record of Deinotheriidae from the Miocene of the Swiss Jura Mountains (Jura Canton, Switzerland) | Fanny Gagliardi, Olivier Maridet, Damien Becker | <p>The Miocene sands of the Swiss Jura Mountains, long exploited in quarries for the construction industry, have yielded abundant fossil remains of large mammals. Among Deinotheriidae (Proboscidea), two species, Prodeinotherium bavaricum and Deino... |  | Fossil record, Paleobiogeography, Taxonomy, Vertebrate paleontology | Lionel Hautier | 2020-08-11 10:17:38 | View | |
25 Oct 2022
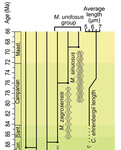
Morphometric changes in two Late Cretaceous calcareous nannofossil lineages support diversification fueled by long-term climate changeMohammad Javad Razmjooei, Nicolas Thibault https://doi.org/10.31233/osf.io/nfyc9Insights into mechanisms of coccolithophore speciation: How useful is cell size in delineating species?Recommended by Emilia Jarochowska based on reviews by Andrej Spiridonov and 1 anonymous reviewer based on reviews by Andrej Spiridonov and 1 anonymous reviewer
Calcareous plankton gives us perhaps the most complete record of microevolutionary changes in the fossil record (e.g. Tong et al., 2018; Weinkauf et al., 2019), but this opportunity is not exploited enough, as it requires meticulous work in documenting assemblage-level variation through time. Especially in organisms such as coccolithophores, understanding the meaning of secular trends in morphology warrants an understanding of the functional biology and ecology of these organisms. Razmjooei and Thibault (2022) achieve this in their painstaking analysis of two coccolithophore lineages, Cribrosphaerella ehrenbergii and Microrhabdulus, in the Late Cretaceous of Iran. They propose two episodes of morphological change. The first one, starting around 76 Ma in the late Campanian, is marked by a sudden shift towards larger sizes of C. ehrenbergii and the appearance of a new species M. zagrosensis from M. undulatus. The second episode around 69 Ma (Maastrichtian) is inferred from a gradual size increase and morphological changes leading to probably anagenetic speciation of M. sinuosus n.sp. The study remarkably analyzed the entire distributions of coccolith length and rod width, rather than focusing on summary statistics (De Baets et al., in press). This is important, because the range of variation determines the taxon’s evolvability with respect to the considered trait (Love et al., 2022). As the authors discuss, cell size in photosymbiotic unicellular organisms is not subject to the same constraints that will be familiar to researchers working e.g. on mammals (Niklas, 1994; Payne et al., 2009; Smith et al., 2016). Furthermore, temporal changes in size alone cannot be interpreted as evolutionary without knowledge of phenotypic plasticity and environmental clines present in the basin (Aloisi, 2015). The more important is that this study cross-tests size changes with other morphological parameters to examine whether their covariation supports inferred speciation events. The article addresses as well the effects of varying sedimentation rates (Hohmann, 2021) by, somewhat implicitly, correcting for the stratophenetic trend using an age-depth model and accounting for a hiatus. Such multifaceted approach as applied in this work is fundamental to unlock the dynamics of speciation offered by the microfossil record. The study highlights also the link between shifts in size and diversity. Klug et al. (2015) have previously demonstrated that these two variables are related, as higher diversity is more likely to lead to extreme values of morphological traits, but this study suggests that the relationship is more intertwined: environmentally-driven rise in morphological variability (and thus in size) can lead to diversification. It is a fantastic illustration of the complexity of morphological evolution that, if it can be evaluated in terms of mechanisms, provides an insight into the dynamics of speciation.
References Aloisi, G. (2015). Covariation of metabolic rates and cell size in coccolithophores. Biogeosciences, 12(15), 4665–4692. doi: 10.5194/bg-12-4665-2015 De Baets, K., Jarochowska, E., Buchwald, S. Z., Klug, C., and Korn, D. (In Press). Lithology controls ammonoid size distribution. Palaios. Hohmann, N. (2021). Incorporating information on varying sedimentation rates into palaeontological analyses. PALAIOS, 36(2), 53–67. doi: 10.2110/palo.2020.038 Klug, C., De Baets, K., Kröger, B., Bell, M. A., Korn, D., and Payne, J. L. (2015). Normal giants? Temporal and latitudinal shifts of Palaeozoic marine invertebrate gigantism and global change. Lethaia, 48(2), 267–288. doi: 10.1111/let.12104 Love, A. C., Grabowski, M., Houle, D., Liow, L. H., Porto, A., Tsuboi, M., Voje, K.L., and Hunt, G. (2022). Evolvability in the fossil record. Paleobiology, 48(2), 186–209. doi: 10.1017/pab.2021.36 Niklas, K. J. (1994). Plant allometry: The scaling of form and process. Chicago: University of Chicago Press. Payne, J. L., Boyer, A. G., Brown, J. H., Finnegan, S., Kowalewski, M., Krause, R. A., Lyons, S.K., McClain, C.R., McShea, D.W., Novack-Gottshall, P.M., Smith, F.A., Stempien, J.A., and Wang, S. C. (2009). Two-phase increase in the maximum size of life over 3.5 billion years reflects biological innovation and environmental opportunity. Proceedings of the National Academy of Sciences, 106(1), 24–27. doi: 10.1073/pnas.0806314106 Razmjooei, M. J., and Thibault, N. (2022). Morphometric changes in two Late Cretaceous calcareous nannofossil lineages support diversification fueled by long-term climate change. PaleorXiv, nfyc9, ver. 4, peer-reviewed by PCI Paleo. doi: 10.31233/osf.io/nfyc9 Smith, F. A., Payne, J. L., Heim, N. A., Balk, M. A., Finnegan, S., Kowalewski, M., Lyons, S.K., McClain, C.R., McShea, D.W., Novack-Gottshall, P.M., Anich, P.S., and Wang, S. C. (2016). Body size evolution across the Geozoic. Annual Review of Earth and Planetary Sciences, 44(1), 523–553. doi: 10.1146/annurev-earth-060115-012147 Tong, S., Gao, K., and Hutchins, D. A. (2018). Adaptive evolution in the coccolithophore Gephyrocapsa oceanica following 1,000 generations of selection under elevated CO2. Global Change Biology, 24(7), 3055–3064. doi: 10.1111/gcb.14065 Weinkauf, M. F. G., Bonitz, F. G. W., Martini, R., and Kučera, M. (2019). An extinction event in planktonic Foraminifera preceded by stabilizing selection. PLOS ONE, 14(10), e0223490. doi: 10.1371/journal.pone.0223490 | Morphometric changes in two Late Cretaceous calcareous nannofossil lineages support diversification fueled by long-term climate change | Mohammad Javad Razmjooei, Nicolas Thibault | <p>Morphometric changes have been investigated in the two groups of calcareous nannofossils, <em>Cribrosphaerella ehrenbergii</em> and <em>Microrhabdulus undosus</em> across the Campanian to Maastrichtian of the Zagros Basin of Iran. Results revea... |  | Biostratigraphy, Evolutionary theory, Fossil record, Microfossils, Micropaleontology, Morphological evolution, Morphometrics, Nanofossils, Paleobiodiversity, Paleobiology, Paleoceanography, Paleoclimatology, Paleoecology, Paleoenvironments, Taxonomy | Emilia Jarochowska | 2020-08-29 12:23:51 | View | |
01 Oct 2021

Ammonoid taxonomy with supervised and unsupervised machine learning algorithmsFloe Foxon https://doi.org/10.31233/osf.io/ewkx9Performance of machine-learning approaches in identifying ammonoid species based on conch propertiesRecommended by Kenneth De Baets based on reviews by Jérémie Bardin and 1 anonymous reviewerThere are less and less experts on taxonomy of particular groups particularly among early career paleontologists and (paleo)biologists – this also includes ammonoid cephalopods. Techniques cannot replace this taxonomic expertise (Engel et al. 2021) but machine learning approaches can make taxonomy more efficient, reproducible as well as passing it over more sustainable. Initially ammonoid taxonomy was a black box with small differences sometimes sufficient to erect different species as well as really idiosyncratic groupings of superficially similar specimens (see De Baets et al. 2015 for a review). In the meantime, scientists have embraced more quantitative assessments of conch shape and morphology more generally (see Klug et al. 2015 for a more recent review). The approaches still rely on important but time-intensive collection work and seeing through daisy chains of more or less accessible papers and monographs without really knowing how these approaches perform (other than expert opinion). In addition, younger scientists are usually trained by more experienced scientists, but this practice is becoming more and more difficult which makes it difficult to resolve the taxonomic gap. This relates to the fact that less and less experienced researchers with this kind of expertise get employed as well as graduate students or postdocs choosing different research or job avenues after their initial training effectively leading to a leaky pipeline and taxonomic impediment. Robust taxonomy and stratigraphy is the basis for all other studies we do as paleontologists/paleobiologists so Foxon (2021) represents the first step to use supervised and unsupervised machine-learning approaches and test their efficiency on ammonoid conch properties. This pilot study demonstrates that machine learning approaches can be reasonably accurate (60-70%) in identifying ammonoid species (Foxon, 2021) – at least similar to that in other mollusk taxa (e.g., Klinkenbuß et al. 2020) - and might also be interesting to assist in cases where more traditional methods are not feasible. Novel approaches might even allow to further approve the accuracy as has been demonstrated for other research objects like pollen (Romero et al. 2020). Further applying of machine learning approaches on larger datasets and additional morphological features (e.g., suture line) are now necessary in order to test and improve the robustness of these approaches for ammonoids as well as test their performance more broadly within paleontology.
References De Baets K, Bert D, Hoffmann R, Monnet C, Yacobucci M, and Klug C (2015). Ammonoid intraspecific variability. In: Ammonoid Paleobiology: From anatomy to ecology. Ed. by Klug C, Korn D, De Baets K, Kruta I, and Mapes R. Vol. 43. Topics in Geobiology. Dordrecht: Springer, pp. 359–426. Engel MS, Ceríaco LMP, Daniel GM, Dellapé PM, Löbl I, Marinov M, Reis RE, Young MT, Dubois A, Agarwal I, Lehmann A. P, Alvarado M, Alvarez N, Andreone F, Araujo-Vieira K, Ascher JS, Baêta D, Baldo D, Bandeira SA, Barden P, Barrasso DA, Bendifallah L, Bockmann FA, Böhme W, Borkent A, Brandão CRF, Busack SD, Bybee SM, Channing A, Chatzimanolis S, Christenhusz MJM, Crisci JV, D’elía G, Da Costa LM, Davis SR, De Lucena CAS, Deuve T, Fernandes Elizalde S, Faivovich J, Farooq H, Ferguson AW, Gippoliti S, Gonçalves FMP, Gonzalez VH, Greenbaum E, Hinojosa-Díaz IA, Ineich I, Jiang J, Kahono S, Kury AB, Lucinda PHF, Lynch JD, Malécot V, Marques MP, Marris JWM, Mckellar RC, Mendes LF, Nihei SS, Nishikawa K, Ohler A, Orrico VGD, Ota H, Paiva J, Parrinha D, Pauwels OSG, Pereyra MO, Pestana LB, Pinheiro PDP, Prendini L, Prokop J, Rasmussen C, Rödel MO, Rodrigues MT, Rodríguez SM, Salatnaya H, Sampaio Í, Sánchez-García A, Shebl MA, Santos BS, Solórzano-Kraemer MM, Sousa ACA, Stoev P, Teta P, Trape JF, Dos Santos CVD, Vasudevan K, Vink CJ, Vogel G, Wagner P, Wappler T, Ware JL, Wedmann S, and Zacharie CK (2021). The taxonomic impediment: a shortage of taxonomists, not the lack of technical approaches. Zoological Journal of the Linnean Society 193, 381–387. doi: 10. 1093/zoolinnean/zlab072 Foxon F (2021). Ammonoid taxonomy with supervised and unsupervised machine learning algorithms. PaleorXiv ewkx9, ver. 3, peer-reviewed by PCI Paleo. doi: 10.31233/osf.io/ewkx9 Klinkenbuß D, Metz O, Reichert J, Hauffe T, Neubauer TA, Wesselingh FP, and Wilke T (2020). Performance of 3D morphological methods in the machine learning assisted classification of closely related fossil bivalve species of the genus Dreissena. Malacologia 63, 95. doi: 10.4002/040.063.0109 Klug C, Korn D, Landman NH, Tanabe K, De Baets K, and Naglik C (2015). Ammonoid conchs. In: Ammonoid Paleobiology: From anatomy to ecology. Ed. by Klug C, Korn D, De Baets K, Kruta I, and Mapes RH. Vol. 43. Dordrecht: Springer, pp. 3–24. Romero IC, Kong S, Fowlkes CC, Jaramillo C, Urban MA, Oboh-Ikuenobe F, D’Apolito C, and Punyasena SW (2020). Improving the taxonomy of fossil pollen using convolutional neural networks and superresolution microscopy. Proceedings of the National Academy of Sciences 117, 28496–28505. doi: 10.1073/pnas.2007324117 | Ammonoid taxonomy with supervised and unsupervised machine learning algorithms | Floe Foxon | <p>Ammonoid identification is crucial to biostratigraphy, systematic palaeontology, and evolutionary biology, but may prove difficult when shell features and sutures are poorly preserved. This necessitates novel approaches to ammonoid taxonomy. Th... |  | Invertebrate paleontology, Taxonomy | Kenneth De Baets | Jérémie Bardin | 2021-01-06 11:48:35 | View |
MANAGING BOARD
Jérémy Anquetin
Faysal Bibi
Guillaume Billet
Andrew A. Farke
Franck Guy
Leslea J. Hlusko
Melanie Hopkins
Cynthia V. Looy
Jesús Marugán-Lobón
Ilaria Mazzini
P. David Polly
Caroline A.E. Strömberg










